Female Sex and Alzheimer's Risk: The Menopause Connection
- PMID: 30298180
- PMCID: PMC6198681
- DOI: 10.14283/jpad.2018.34
Female Sex and Alzheimer's Risk: The Menopause Connection
Abstract
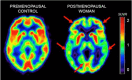
Along with advanced age and apolipoprotein E (APOE)-4 genotype, female sex is a major risk factor for developing late-onset Alzheimer's disease (AD). Considering that AD pathology begins decades prior to clinical symptoms, the higher risk in women cannot simply be accounted for by their greater longevity as compared to men. Recent investigation into sex-specific pathophysiological mechanisms behind AD risk has implicated the menopause transition (MT), a midlife neuroendocrine transition state unique to females. Commonly characterized as ending in reproductive senescence, many symptoms of MT are neurological, including disruption of estrogen-regulated systems such as thermoregulation, sleep, and circadian rhythms, as well as depression and impairment in multiple cognitive domains. Preclinical studies have shown that, during MT, the estrogen network uncouples from the brain bioenergetic system. The resulting hypometabolic state could serve as the substrate for neurological dysfunction. Indeed, translational brain imaging studies demonstrate that 40-60 year-old perimenopausal and postmenopausal women exhibit an AD-endophenotype characterized by decreased metabolic activity and increased brain amyloid-beta deposition as compared to premenopausal women and to age-matched men. This review discusses the MT as a window of opportunity for therapeutic interventions to compensate for brain bioenergetic crisis and combat the subsequent increased risk for AD in women.
Keywords: Alzheimer’s disease; Perimenopause; brain imaging; early detection; women.
Conflict of interest statement
The authors declare no disclosures.
Figures
References
-
- Farrer L.A., et al. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. JAMA. 1997;278(16):1349–1356. 10.1001/jama.1997.03550160069041 PubMed PMID: 9343467. - DOI - PubMed
-
- Alzheimer's A. 2016 Alzheimer's disease facts and figures. Alzheimers Dement. 2016;12(4):459–509. 10.1016/j.jalz.2016.03.001 - DOI - PubMed
-
- Stopping Alzheimer's Disease and Related Dementias: Advancing Our Nation's Research AgendaNIH Bypass Budget Proposal for Fiscal Year 2018 N.I.o. Health, Editor. 2018.
-
- Vina J., Lloret A. Why women have more Alzheimer's disease than men: gender and mitochondrial toxicity of amyloid-beta peptide. J Alzheimers Dis. 2010;20(2):S527–S533. 10.3233/JAD-2010-100501 PubMed PMID: 20442496. - DOI - PubMed
-
- Brinton R.D., et al. Perimenopause as a neurological transition state. Nat Rev Endocrinol. 2015;11(7):393–405. 10.1038/nrendo.2015.82 PubMed PMID: 26007613. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous