Assessment of Th1/Th2 Bias of STING Agonists Coated on Microneedles for Possible Use in Skin Allergen Immunotherapy
- PMID: 30299105
- PMCID: PMC6857785
- DOI: 10.1021/acs.molpharmaceut.8b00768
Assessment of Th1/Th2 Bias of STING Agonists Coated on Microneedles for Possible Use in Skin Allergen Immunotherapy
Abstract
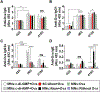
Microneedle-based skin allergen-specific immunotherapy (AIT) can benefit from adjuvants that can stimulate a stronger Th1 response against the allergen. We evaluated two stimulator of interferon genes (STING) agonists, namely, cyclic diguanylate monophosphate (c-di-GMP) and cyclic diadenylate monophosphate (c-di-AMP), as skin adjuvants using coated microneedles (MNs). For comparison, the approved subcutaneous (SC) hypodermic injection containing alum was used. Ovalbumin (Ova) was used as a model allergen. Ova-specific IgG2a antibody in serum, which is a surrogate marker for Th1 type immune response was significantly higher when STING agonists were used with coated MNs as compared to SC injection of Ova+alum in mice. In contrast, IgG1 antibody, a surrogate marker for Th2 type immune response, was at comparable levels in the MN and SC groups. Restimulation of splenocytes with Ova produced higher levels of Th1 cytokines (IFN-γ and IL-2) in the STING agonists MN groups as compared to the SC group. In conclusion, delivery of STING agonists into the skin using coated MNs activated the Th1 pathway better than SC- and MN-based delivery of alum. Thus, STING agonists could fulfill the role of adjuvants for skin AIT and even for infectious disease vaccines, where stimulation of the Th1 pathway is of interest.
Keywords: STING adjuvants; allergy adjuvant; cutaneous-allergen immunotherapy; microneedles; skin immunotherapy.
Conflict of interest statement
Conflict of interest
HSG and AKS are co-inventors on a patent related to coated microneedles for allergen immunotherapy. HSG is also part of a startup company that is developing microneedles for food allergy immunotherapy. These potential conflicts of interest have been disclosed and are being managed by Texas Tech University.
Figures



Similar articles
-
Microneedle-Mediated Allergen-Specific Immunotherapy for the Treatment of Airway Allergy in Mice.Mol Pharm. 2020 Aug 3;17(8):3033-3042. doi: 10.1021/acs.molpharmaceut.0c00447. Epub 2020 Jul 23. Mol Pharm. 2020. PMID: 32643940
-
A comparative study of microneedle-based cutaneous immunization with other conventional routes to assess feasibility of microneedles for allergy immunotherapy.Vaccine. 2015 Aug 7;33(33):4060-4. doi: 10.1016/j.vaccine.2015.06.042. Epub 2015 Jun 16. Vaccine. 2015. PMID: 26092307
-
Cutaneous vaccination with coated microneedles prevents development of airway allergy.J Control Release. 2017 Nov 10;265:75-82. doi: 10.1016/j.jconrel.2017.08.012. Epub 2017 Aug 15. J Control Release. 2017. PMID: 28821461 Free PMC article.
-
Deviation of the allergic IgE to an IgG response by gene immunotherapy.Int Rev Immunol. 1999;18(3):271-89. doi: 10.3109/08830189909043030. Int Rev Immunol. 1999. PMID: 10614729 Review.
-
Allergy vaccines--new approaches to an old concept.Expert Opin Biol Ther. 2004 Sep;4(9):1473-81. doi: 10.1517/14712598.4.9.1473. Expert Opin Biol Ther. 2004. PMID: 15335314 Review.
Cited by
-
Transdermal delivery for gene therapy.Drug Deliv Transl Res. 2022 Nov;12(11):2613-2633. doi: 10.1007/s13346-022-01138-1. Epub 2022 May 10. Drug Deliv Transl Res. 2022. PMID: 35538189 Free PMC article. Review.
-
cGAMP/Saponin Adjuvant Combination Improves Protective Response to Influenza Vaccination by Microneedle Patch in an Aged Mouse Model.Front Immunol. 2021 Feb 2;11:583251. doi: 10.3389/fimmu.2020.583251. eCollection 2020. Front Immunol. 2021. PMID: 33603732 Free PMC article.
-
Gene delivery for immunoengineering.Curr Opin Biotechnol. 2020 Dec;66:1-10. doi: 10.1016/j.copbio.2020.05.008. Epub 2020 Jun 15. Curr Opin Biotechnol. 2020. PMID: 32554325 Free PMC article. Review.
-
Microneedles coated with peanut allergen enable desensitization of peanut sensitized mice.J Control Release. 2019 Nov 28;314:38-47. doi: 10.1016/j.jconrel.2019.09.022. Epub 2019 Oct 15. J Control Release. 2019. PMID: 31626861 Free PMC article.
-
Approaches and applications in transdermal and transpulmonary gene drug delivery.Front Bioeng Biotechnol. 2025 Jan 15;12:1519557. doi: 10.3389/fbioe.2024.1519557. eCollection 2024. Front Bioeng Biotechnol. 2025. PMID: 39881959 Free PMC article. Review.
References
-
- Passalacqua G; Bagnasco D; Ferrando M; Heffler E; Puggioni F; Canonica GW Current insights in allergen immunotherapy. Ann. Allergy Asthma Immunol. 2018, 120, (2), 152–154. - PubMed
-
- Cox L; Nelson H; Lockey R; Calabria C; Chacko T; Finegold I; Nelson M; Weber R; Bernstein DI; Blessing-Moore J; Khan DA; Lang DM; Nicklas RA; Oppenheimer J; Portnoy JM; Randolph C; Schuller DE; Spector SL; Tilles S; Wallace D Allergen immunotherapy: a practice parameter third update. J. Allergy Clin. Immunol. 2011, 127, (1 Suppl), S1–55. - PubMed
-
- Senna G; Ridolo E; Calderon M; Lombardi C; Canonica GW; Passalacqua G Evidence of adherence to allergen-specific immunotherapy. Curr. Opin. Allergy Clin. Immunol. 2009, 9, (6), 544–8. - PubMed
-
- Shakya AK; Gill HS A comparative study of microneedle-based cutaneous immunization with other conventional routes to assess feasibility of microneedles for allergy immunotherapy. Vaccine 2015, 33, (33), 4060–4. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

