Genome-Wide Association Study of Susceptibility Loci for Radiation-Induced Brain Injury
- PMID: 30299488
- PMCID: PMC6579742
- DOI: 10.1093/jnci/djy150
Genome-Wide Association Study of Susceptibility Loci for Radiation-Induced Brain Injury
Abstract
Background: Radiation-induced brain injury is a nonnegligible issue in the management of cancer patients treated by partial or whole brain irradiation. In particular, temporal lobe injury (TLI), a deleterious late complication in nasopharyngeal carcinoma, greatly affects the long-term life quality of these patients. Although genome-wide association studies (GWASs) have successfully identified single nucleotide polymorphisms (SNPs) associated with radiation toxicity, genetic variants contributing to the radiation-induced brain injury have not yet been assessed.
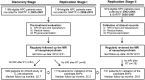
Methods: We recruited and performed follow-up for a prospective observational cohort, Genetic Architecture of Radiotherapy Toxicity and Prognosis, using magnetic resonance imaging for TLI diagnosis. We conducted genome-wide association analysis in 1082 patients and validated the top associations in two independent cohorts of 1119 and 741 patients, respectively. All statistical tests were two-sided.
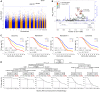
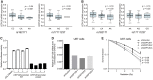
Results: We identified a promoter variant rs17111237 (A > G, minor allele frequency [MAF] = 0.14) in CEP128 associated with TLI risk (hazard ratio = 1.45, 95% confidence interval = 1.26 to 1.66, Pcombined=3.18 × 10-7) which is in moderate linkage disequilibrium (LD) with rs162171 (MAF = 0.18, R2 = 0.69), the top signal in CEP128 (hazard ratio = 1.46, 95% confidence interval = 1.29-1.66, Pcombined= 6.17 × 10-9). Combining the clinical variables with the top SNP, we divided the patients into different subgroups with varying risk with 5-year TLI-free rates ranging from 33.7% to 95.5%. CEP128, a key component of mother centriole, tightly interacts with multiple radiation-resistant genes and plays an important role in maintaining the functional cilia, which otherwise will lead to a malfunction of the neural network. We found that A > G alteration at rs17111237 impaired the promoter activity of CEP128 and knockdown of CEP128 decreased the clonogenic cell survival of U87 cells under radiation. Noteworthy, 12.7% (27/212) of the GWAS-based associated genes (P < .001) were enriched in the neurogenesis pathway.
Conclusions: This three-stage study is the first GWAS of radiation-induced brain injury that implicates the genetic susceptibility gene CEP128 involved in TLI development and provides the novel insight into the underlying mechanisms of radiation-induced brain injury.
© The Author(s) 2018. Published by Oxford University Press.
Figures
References
-
- Lee AW, Law SC, Ng SH, et al. Retrospective analysis of nasopharyngeal carcinoma treated during 1976-1985: late complications following megavoltage irradiation. Br J Radiol. 1992;65778:918–928. - PubMed
-
- Wang YX, King AD, Zhou H, et al. Evolution of radiation-induced brain injury: MR imaging-based study. Radiology. 2010;2541:210–218. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

