Order in Disorder as Observed by the "Hydrophobic Cluster Analysis" of Protein Sequences
- PMID: 30299594
- PMCID: PMC7168002
- DOI: 10.1002/pmic.201800054
Order in Disorder as Observed by the "Hydrophobic Cluster Analysis" of Protein Sequences
Abstract
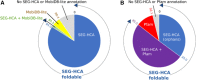
Hydrophobic cluster analysis (HCA) is an original approach for protein sequence analysis, which provides access to the foldable repertoire of the protein universe, including yet unannotated protein segments ("dark proteome"). Foldable segments correspond to ordered regions, as well as to intrinsically disordered regions (IDRs) undergoing disorder to order transitions. In this review, how HCA can be used to give insight into this last category of foldable segments is illustrated, with examples matching known 3D structures. After reviewing the HCA principles, examples of short foldable segments are given, which often contain short linear motifs, typically matching hydrophobic clusters. These segments become ordered upon contact with partners, with secondary structure preferences generally corresponding to those observed in the 3D structures within the complexes. Such small foldable segments are sometimes larger than the segments of known 3D structures, including flanking hydrophobic clusters that may be critical for interaction specificity or regulation, as well as intervening sequences allowing fuzziness. Cases of larger conditionally disordered domains are also presented, with lower density in hydrophobic clusters than well-folded globular domains or with exposed hydrophobic patches, which are stabilized by interaction with partners.
Keywords: HCA; dark proteome; disorder; foldability; secondary structure.
© 2018 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Jin J., Xie X., Chen C., Park J. G., Stark C., James D. A., Olhovsky M., Linding R., Mao Y., Pawson T., Sci. Signal 2009, 2, ra76. - PubMed
-
- Bornberg‐Bauer E., Albà M. M., Curr. Opin. Struct. Biol. 2013, 23, 459. - PubMed
-
- Forslund K., Henricson A., Hollich V., Sonnhammer E. L., Mol. Biol. Evol. 2008, 25, 254. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

