Intrinsic activation of the vitamin D antimicrobial pathway by M. leprae infection is inhibited by type I IFN
- PMID: 30300363
- PMCID: PMC6177120
- DOI: 10.1371/journal.pntd.0006815
Intrinsic activation of the vitamin D antimicrobial pathway by M. leprae infection is inhibited by type I IFN
Abstract
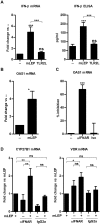
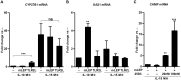
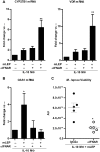
Following infection, virulent mycobacteria persist and grow within the macrophage, suggesting that the intrinsic activation of an innate antimicrobial response is subverted by the intracellular pathogen. For Mycobacterium leprae, the intracellular bacterium that causes leprosy, the addition of exogenous innate or adaptive immune ligands to the infected monocytes/macrophages was required to detect a vitamin D-dependent antimicrobial activity. We investigated whether there is an intrinsic immune response to M. leprae in macrophages that is inhibited by the pathogen. Upon infection of monocytes with M. leprae, there was no upregulation of CYP27B1 nor its enzymatic activity converting the inactive prohormone form of vitamin D (25-hydroxyvitamin D) to the bioactive form (1,25α-dihydroxyvitamin D). Given that M. leprae-induced type I interferon (IFN) inhibited monocyte activation, we blocked the type I IFN receptor (IFNAR), revealing the intrinsic capacity of monocytes to recognize M. leprae and upregulate CYP27B1. Consistent with these in vitro studies, an inverse relationship between expression of CYP27B1 vs. type I IFN downstream gene OAS1 was detected in leprosy patient lesions, leading us to study cytokine-derived macrophages (MΦ) to model cellular responses at the site of disease. Infection of IL-15-derived MΦ, similar to MΦ in lesions from the self-limited form of leprosy, with M. leprae did not inhibit induction of the vitamin D antimicrobial pathway. In contrast, infection of IL-10-derived MΦ, similar to MΦ in lesions from patients with the progressive form of leprosy, resulted in induction of type I IFN and suppression of the vitamin D directed pathway. Importantly, blockade of the type I IFN response in infected IL-10 MΦ decreased M. leprae viability. These results indicate that M. leprae evades the intrinsic capacity of human monocytes/MΦ to activate the vitamin D-mediated antimicrobial pathway via the induction of type I IFN.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

