Hybrid topoisomerase I and HDAC inhibitors as dual action anticancer agents
- PMID: 30300374
- PMCID: PMC6177136
- DOI: 10.1371/journal.pone.0205018
Hybrid topoisomerase I and HDAC inhibitors as dual action anticancer agents
Abstract
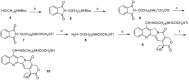
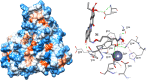
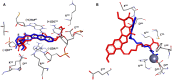
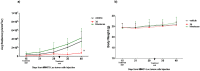
Recent studies have shown that HDAC inhibitors act synergistically with camptothecin derivatives in combination therapies. To exploit this synergy, new hybrid molecules targeting simultaneously topoisomerase I and HDAC were designed. In particular, a selected multivalent agent containing a camptothecin and a SAHA-like template showed a broad spectrum of antiproliferative activity, with IC50 values in the nanomolar range. Preliminary in vivo results indicated a strong antitumor activity on human mesothelioma primary cell line MM473 orthotopically xenografted in CD-1 nude mice and very high tolerability.
Conflict of interest statement
The authors have the following interests. Scientia Advice is the name of Dr. Roberto Artali's sole proprietorship who offered a free-of-charge cooperation in the molecular modelling. There are no patents, products in development or marketed products to declare. This does not alter the authors' adherence to all the PLOS ONE policies on sharing data and materials, as detailed online in the guide for authors.
Figures






References
-
- Sundar R, Valeri N, Harrington KJ, Yap TA. Clin. Combining Molecularly Targeted Agents: Is More Always Better? Cancer Res. 2017; 23: 1123–1125. - PubMed
-
- Abbot V, Sharma P, Dhiman S, Noolvi MN, Patel HM, Bhardwaj V. Small hybrid heteroaromatics: resourceful biological tools in cancer research. RSC Advances 2017; 7: 28313–28349.

