In Vitro Flow Rate Dependency of Delivered Dose and Fine Particle Dose of Salmeterol/Fluticasone Propionate Easyhaler and Seretide Diskus with Patient Flow Rates Collected in a Randomized Controlled Trial
- PMID: 30300557
- PMCID: PMC6477585
- DOI: 10.1089/jamp.2018.1463
In Vitro Flow Rate Dependency of Delivered Dose and Fine Particle Dose of Salmeterol/Fluticasone Propionate Easyhaler and Seretide Diskus with Patient Flow Rates Collected in a Randomized Controlled Trial
Abstract
Background: The Easyhaler® device-metered dry powder inhaler containing Salmeterol and Fluticasone propionate (S/F) has been developed for the treatment of patients with asthma and chronic obstructive pulmonary disease (COPD). We report two studies which evaluated the in vitro flow rate dependence of delivered dose (DD) and fine particle dose (FPD) of S/F Easyhaler versus Seretide Diskus®.
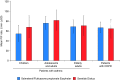
Methods: A randomized controlled trial (RCT) assessed inspiratory flow parameters of S/F Easyhaler and Seretide Diskus in subgroups of patients with asthma (children, adolescents and adults, and elderly) and in COPD patients. The 10th, 50th, and 90th percentile airflow rates were determined and utilized in vitro, to evaluate flow rate dependence of DD and FPD. Flow rate dependence was evaluated relative to the result obtained at the 50th percentile and any values deviating from 100% indicated flow rate dependence. The volumetric flow rate dependence (Q) index derived from FPD at 10th and 90th percentile airflows was also evaluated.
Results: Overall, 227 patients were enrolled and randomized; 216 completed the RCT. In total, 55.5% of patients were female, and the mean age was 46.3 years. Clinically relevant airflow rates (46, 68, and 85 L/min for S/F Easyhaler and 44, 71, and 96 L/min for Seretide Diskus) were carried forward into the in vitro study, which demonstrated similar flow rate dependence of DD and FPD for S/F Easyhaler compared with Seretide Diskus; all values were within ±15% limits across the 10th, 50th, and 90th percentile airflow rates. Q index results suggested that both S/F Easyhaler and Seretide Diskus are medium airflow-dependent products.
Conclusions: Similar in vitro flow rate dependence of DD and FPD was demonstrated for S/F Easyhaler compared with Seretide Diskus, across a range of clinically relevant airflow rates, collected from patients with asthma and COPD.
Keywords: Salmeterol/Fluticasone Easyhaler; delivered dose; fine particle dose; flow rate dependency.
Conflict of interest statement
S.L., M.V., A.H., and J.H. are employees of Orion Corporation, Orion Pharma. R.J. reports no conflicts of interest.
Figures




Similar articles
-
Consistent Dosing Through the Salmeterol-Fluticasone Propionate Easyhaler for the Management of Asthma and Chronic Obstructive Pulmonary Disease: Robustness Analysis Across the Easyhaler Lifetime.J Aerosol Med Pulm Drug Deliv. 2021 Jun;34(3):189-196. doi: 10.1089/jamp.2020.1592. Epub 2020 Sep 22. J Aerosol Med Pulm Drug Deliv. 2021. PMID: 32960127 Free PMC article.
-
Comparative Analysis of Persistence to Treatment among Patients with Asthma or COPD Receiving AirFluSal Forspiro or Seretide Diskus Salmeterol/Fluticasone Propionate Combination Therapy.J Allergy Clin Immunol Pract. 2016 Sep-Oct;4(5):884-9. doi: 10.1016/j.jaip.2016.07.006. J Allergy Clin Immunol Pract. 2016. PMID: 27587319
-
Pharmacokinetics and pharmacodynamics of fluticasone propionate and salmeterol delivered as a combination dry powder from a capsule-based inhaler and a multidose inhaler in asthma and COPD patients.J Aerosol Med Pulm Drug Deliv. 2014 Aug;27(4):279-89. doi: 10.1089/jamp.2013.1040. Epub 2013 Sep 28. J Aerosol Med Pulm Drug Deliv. 2014. PMID: 24074143 Clinical Trial.
-
Fluticasone Propionate/Salmeterol MDPI (AirDuo RespiClick®): A Review in Asthma.Clin Drug Investig. 2018 May;38(5):463-473. doi: 10.1007/s40261-018-0644-2. Clin Drug Investig. 2018. PMID: 29582249 Review.
-
Seretide for obstructive lung disease.Expert Opin Pharmacother. 2002 Mar;3(3):341-50. doi: 10.1517/14656566.3.3.341. Expert Opin Pharmacother. 2002. PMID: 11866683 Review.
Cited by
-
A non-interventional switch study in adult patients with asthma or COPD on clinical effectiveness of salmeterol/fluticasone Easyhaler® in routine clinical practice.Ther Adv Respir Dis. 2021 Jan-Dec;15:17534666211027787. doi: 10.1177/17534666211027787. Ther Adv Respir Dis. 2021. PMID: 34344257 Free PMC article.
-
Dry-powder inhaler use in primary school-aged children with asthma: a systematic review.ERJ Open Res. 2024 Dec 9;10(6):00455-2024. doi: 10.1183/23120541.00455-2024. eCollection 2024 Nov. ERJ Open Res. 2024. PMID: 39655170 Free PMC article.
-
Lung Deposition and Inspiratory Flow Rate in Patients with Chronic Obstructive Pulmonary Disease Using Different Inhalation Devices: A Systematic Literature Review and Expert Opinion.Int J Chron Obstruct Pulmon Dis. 2021 Apr 19;16:1021-1033. doi: 10.2147/COPD.S297980. eCollection 2021. Int J Chron Obstruct Pulmon Dis. 2021. PMID: 33907390 Free PMC article.
-
Patients with asthma or chronic obstructive pulmonary disease (COPD) can generate sufficient inspiratory flows via Easyhaler® dry powder inhaler: a pooled analysis of two randomized controlled trials.J Thorac Dis. 2021 Feb;13(2):621-631. doi: 10.21037/jtd-20-2112. J Thorac Dis. 2021. PMID: 33717535 Free PMC article.
-
Understanding Dry Powder Inhalers: Key Technical and Patient Preference Attributes.Adv Ther. 2019 Oct;36(10):2547-2557. doi: 10.1007/s12325-019-01066-6. Epub 2019 Sep 2. Adv Ther. 2019. PMID: 31478131 Free PMC article. Review.
References
-
- Magnussen H: Novolizer: How does it fit into inhalation therapy? Curr Med Res Opin. 2005;21 Suppl 4:S39–S46; discussion S47. - PubMed
-
- Melani AS: Inhalatory therapy training: A priority challenge for the physician. Acta Biomed. 2007;78:233–245 - PubMed
-
- Committee for Medicinal Products for Human Use: Guideline on the requirements for clinical documentation for orally inhaled products (OIP) including the requirements for demonstration of therapeutic equivalence between the two inhaled products for use in the treatment of asthma and chronic obstructive pulmonary disease (COPD) in adults and for use in the treatment of asthma in children and adolescents. 2009. Available at: www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/... Accessed September27, 2017
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
