Knockdown of LXRα Inhibits Goat Intramuscular Preadipocyte Differentiation
- PMID: 30301149
- PMCID: PMC6213902
- DOI: 10.3390/ijms19103037
Knockdown of LXRα Inhibits Goat Intramuscular Preadipocyte Differentiation
Abstract
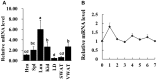
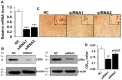
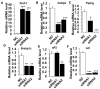
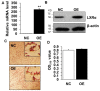
Goat intramuscular fat (IMF) content is mainly determined by the processes of intramuscular preadipocytes adipogenic differentiation and mature adipocyte lipid accumulation. However, the underlying regulators of these biological processes remain largely unknown. Here, we report that the expression of Liver X receptor alpha (LXRα) reaches a peak at early stage and then gradually decreases during goat intramuscular adipogenesis. Knockdown of LXRα mediated by two independent siRNAs significantly inhibits intramuscular adipocytes lipid accumulation and upregulates preadipocytes marker- preadipocyte factor 1 (pref1) expression. Consistently, siRNA treatments robustly decrease mRNA level of adipogenic related genes, including CCAAT enhancer binding protein alpha (Cebpα), Peroxisome proliferator activated receptor gamma (Pparg), Sterol regulatory element binding protein isoform 1c (Srebp1c), Fatty acids binding protein (aP2) and Lipoprotein lipase (Lpl). Next, adenovirus overexpression of LXRα does not affect intramuscular adipocytes adipogenesis manifested by Oil Red O signal measurement and adipogenic specific genes detection. Mechanically, we found that both CCAAT enhancer binding protein beta (Cebpβ) and Kruppel like factor 8 (Klf8) are potential targets of LXRα, indicated by having putative binding sites of LXRα at the promoter of these genes and similar expression pattern during adipogenesis comparing to LXRα. Importantly, mRNA levels of Cebpβ and Klf8 are downregulated significantly in goat LXRα knockdown intramuscular adipocyte. These results demonstrate that loss function of LXRα inhibits intramuscular adipogenesis possibly through down-regulation of Cebpβ and Klf8. Our research will provide new insights into mechanical regulation of goat IMF deposition.
Keywords: Capra hircus; LXRα; goat; intramuscular adipocyte; intramuscular fat.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Cheng W.W., Cheng J.H., Sun D.W., Pu H.B. Marbling Analysis for Evaluating Meat Quality: Methods and Techniques. Compr. Rev. Food Sci. Food Saf. 2015;14:523–535. doi: 10.1111/1541-4337.12149. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

