Microbiota and Phage Therapy: Future Challenges in Medicine
- PMID: 30301167
- PMCID: PMC6313512
- DOI: 10.3390/medsci6040086
Microbiota and Phage Therapy: Future Challenges in Medicine
Abstract
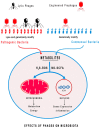
An imbalance of bacterial quantity and quality of gut microbiota has been linked to several pathologies. New strategies of microbiota manipulation have been developed such as fecal microbiota transplantation (FMT); the use of pre/probiotics; an appropriate diet; and phage therapy. The presence of bacteriophages has been largely underestimated and their presence is a relevant component for the microbiome equilibrium. As a promising treatment, phage therapy has been extensively used in Eastern Europe to reduce pathogenic bacteria and has arisen as a new method to modulate microbiota diversity. Phages have been selected and "trained" to infect a wide spectrum of bacteria or tailored to infect specific antibiotic resistant bacteria present in patients. The new development of genetically modified phages may be an efficient tool to treat the gut microbiota dysbiosis associated with different pathologies and increased production of bacterial metabolites and subsequently decrease systemic low-grade chronic inflammation associated with chronic diseases. Microbiota quality and mitochondria dynamics can be remodulated and manipulated by phages to restore the equilibrium and homeostasis of the system. Our aim is to highlight the great interest for phages not only to eliminate and control pathogenic bacterial infection but also in the near future to modulate the microbiota by adding new functions to selected bacteria species and rebalance the dynamic among phages and bacteria. The challenge for the medicine of tomorrow is to re-think and redesign strategies differently and far from our traditional thinking.
Keywords: Phage therapy; chronic disease; microbiota; mitochondria; pathology treatment.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Kieser S., Sarker S.A., Sakwinska O., Foata F., Sultana S., Khan Z., Islam S., Porta N., Combremont S., Betrisey B., et al. Bangladeshi children with acute diarrhea show fecal microbiomes with increased Streptococcus abundance, irrespective of diarrhea etiology. Environ. Microbiol. 2018 doi: 10.1111/1462-2920.14274. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

