Secondary Unconjugated Bile Acids Induce Hepatic Stellate Cell Activation
- PMID: 30301191
- PMCID: PMC6213941
- DOI: 10.3390/ijms19103043
Secondary Unconjugated Bile Acids Induce Hepatic Stellate Cell Activation
Abstract
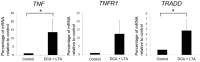
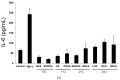
Hepatic stellate cells (HSCs) are key players in liver fibrosis, cellular senescence, and hepatic carcinogenesis. Bile acids (BAs) are involved in the activation of HSCs, but the detailed mechanism of this process remains unclear. We conducted a comprehensive DNA microarray study of the human HSC line LX-2 treated with deoxycholic acid (DCA), a secondary unconjugated BA. Additionally, LX-2 cells were exposed to nine BAs and studied using immunofluorescence staining, enzyme-linked immunosorbent assay, and flow cytometry to examine the mechanisms of HSC activation. We focused on the tumor necrosis factor (TNF) pathway and revealed upregulation of genes related to nuclear factor kappa B (NF-κB) signaling and senescence-associated secretory phenotype factors. α-Smooth muscle actin (α-SMA) was highly expressed in cells treated with secondary unconjugated BAs, including DCA, and a morphological change associated with radial extension of subendothelial protrusion was observed. Interleukin-6 level in culture supernatant was significantly higher in cells treated with secondary unconjugated BAs. Flow cytometry showed that the proportion of cells highly expressing α-SMA was significantly increased in HSCs cultured with secondary unconjugated BAs. We demonstrated that secondary unconjugated BAs induced the activation of human HSCs.
Keywords: DNA microarray; hepatic stellate cell; secondary unconjugated bile acid; tumor necrosis factor signaling pathway.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures







Similar articles
-
Newcastle disease virus represses the activation of human hepatic stellate cells and reverses the development of hepatic fibrosis in mice.Liver Int. 2009 Apr;29(4):593-602. doi: 10.1111/j.1478-3231.2009.01971.x. Epub 2009 Jan 28. Liver Int. 2009. PMID: 19192169
-
Guggulsterone attenuates activation and survival of hepatic stellate cell by inhibiting nuclear factor kappa B activation and inducing apoptosis.J Gastroenterol Hepatol. 2013 Dec;28(12):1859-68. doi: 10.1111/jgh.12314. J Gastroenterol Hepatol. 2013. PMID: 23808824
-
Tetrandrine regulates hepatic stellate cell activation via TAK1 and NF-κB signaling.Int Immunopharmacol. 2016 Jul;36:263-270. doi: 10.1016/j.intimp.2016.04.039. Epub 2016 May 12. Int Immunopharmacol. 2016. PMID: 27179306
-
2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) induces hepatic stellate cell (HSC) activation and liver fibrosis in C57BL6 mouse via activating Akt and NF-κB signaling pathways.Toxicol Lett. 2017 May 5;273:10-19. doi: 10.1016/j.toxlet.2017.03.013. Epub 2017 Mar 14. Toxicol Lett. 2017. PMID: 28302560
-
Role of bile acids in colon carcinogenesis.World J Clin Cases. 2018 Nov 6;6(13):577-588. doi: 10.12998/wjcc.v6.i13.577. World J Clin Cases. 2018. PMID: 30430113 Free PMC article. Review.
Cited by
-
GRP94 Inhabits the Immortalized Porcine Hepatic Stellate Cells Apoptosis under Endoplasmic Reticulum Stress through Modulating the Expression of IGF-1 and Ubiquitin.Int J Mol Sci. 2022 Nov 14;23(22):14059. doi: 10.3390/ijms232214059. Int J Mol Sci. 2022. PMID: 36430538 Free PMC article.
-
Senescence in HBV-, HCV- and NAFLD- Mediated Hepatocellular Carcinoma and Senotherapeutics: Current Evidence and Future Perspective.Cancers (Basel). 2021 Sep 21;13(18):4732. doi: 10.3390/cancers13184732. Cancers (Basel). 2021. PMID: 34572959 Free PMC article. Review.
-
Chronic-binge ethanol feeding aggravates systemic dyslipidemia in Ldlr-/- mice, thereby accelerating hepatic fibrosis.Front Endocrinol (Lausanne). 2023 Jul 25;14:1148827. doi: 10.3389/fendo.2023.1148827. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37560305 Free PMC article.
-
Emerging role of bile acids in colorectal liver metastasis: From molecular mechanism to clinical significance (Review).Int J Oncol. 2025 Mar;66(3):24. doi: 10.3892/ijo.2025.5730. Epub 2025 Feb 21. Int J Oncol. 2025. PMID: 39981904 Free PMC article. Review.
-
Senescent hepatic stellate cells caused by deoxycholic acid modulates malignant behavior of hepatocellular carcinoma.J Cancer Res Clin Oncol. 2020 Dec;146(12):3255-3268. doi: 10.1007/s00432-020-03374-9. Epub 2020 Sep 1. J Cancer Res Clin Oncol. 2020. PMID: 32870388 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

