Neratinib is effective in breast tumors bearing both amplification and mutation of ERBB2 (HER2)
- PMID: 30301790
- PMCID: PMC6498841
- DOI: 10.1126/scisignal.aat9773
Neratinib is effective in breast tumors bearing both amplification and mutation of ERBB2 (HER2)
Abstract
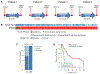
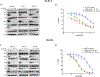
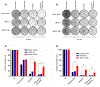
Mutations in ERBB2, the gene encoding epidermal growth factor receptor (EGFR) family member HER2, are common in and drive the growth of "HER2-negative" (not ERBB2 amplified) tumors but are rare in "HER2-positive" (ERBB2 amplified) breast cancer. We analyzed DNA-sequencing data from HER2-positive patients and used cell lines and a patient-derived xenograft model to test the consequence of HER2 mutations on the efficacy of anti-HER2 agents such as trastuzumab, lapatinib, and neratinib, an irreversible pan-EGFR inhibitor. HER2 mutations were present in ~7% of HER2-positive tumors, all of which were metastatic but not all were previously treated. Compared to HER2 amplification alone, in both patients and cultured cell lines, the co-occurrence of HER2 mutation and amplification was associated with poor response to trastuzumab and lapatinib, the standard-of-care anti-HER2 agents. In mice, xenografts established from a patient whose HER2-positive tumor acquired a D769Y mutation in HER2 after progression on trastuzumab-based therapy were resistant to trastuzumab or lapatinib but were sensitive to neratinib. Clinical data revealed that six heavily pretreated patients with tumors bearing coincident HER2 amplification and mutation subsequently exhibited a statistically significant response to neratinib monotherapy. Thus, these findings indicate that coincident HER2 mutation reduces the efficacy of therapies commonly used to treat HER2-positive breast cancer, particularly in metastatic and previously HER2 inhibitor-treated patients, as well as potentially in patients scheduled for first-line treatment. Therefore, we propose that clinical studies testing the efficacy of neratinib are warranted selectively in breast cancer patients whose tumors carry both amplification and mutation of ERBB2/HER2.
Copyright © 2018 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Conflict of interest statement
Figures


References
-
- Montemurro F, Scaltriti M, Biomarkers of drugs targeting HER-family signalling in cancer. The Journal of pathology 232, 219–229 (2014). - PubMed
-
- Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL, Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 235, 177–182 (1987). - PubMed
-
- Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE, Levin WJ, Stuart SG, Udove J, Ullrich A, et al., Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science 244, 707–712 (1989). - PubMed
-
- Serra V, Vivancos A, Puente XS, Felip E, Silberschmidt D, Caratu G, Parra JL, De Mattos-Arruda L, Grueso J, Hernandez-Losa J, Arribas J, Prudkin L, Nuciforo P, Scaltriti M, Seoane J, Baselga J, Clinical response to a lapatinib-based therapy for a Li-Fraumeni syndrome patient with a novel HER2V659 E mutation. Cancer discovery 3, 1238–1244 (2013). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

