The oral microbiota: dynamic communities and host interactions
- PMID: 30301974
- PMCID: PMC6278837
- DOI: 10.1038/s41579-018-0089-x
The oral microbiota: dynamic communities and host interactions
Abstract
The dynamic and polymicrobial oral microbiome is a direct precursor of diseases such as dental caries and periodontitis, two of the most prevalent microbially induced disorders worldwide. Distinct microenvironments at oral barriers harbour unique microbial communities, which are regulated through sophisticated signalling systems and by host and environmental factors. The collective function of microbial communities is a major driver of homeostasis or dysbiosis and ultimately health or disease. Despite different aetiologies, periodontitis and caries are each driven by a feedforward loop between the microbiota and host factors (inflammation and dietary sugars, respectively) that favours the emergence and persistence of dysbiosis. In this Review, we discuss current knowledge and emerging mechanisms governing oral polymicrobial synergy and dysbiosis that have both enhanced our understanding of pathogenic mechanisms and aided the design of innovative therapeutic approaches for oral diseases.
Conflict of interest statement
Competing interests
The authors declare no competing interests.
Figures
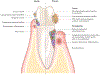
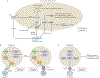

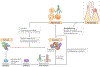
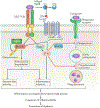
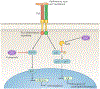
References
-
-
Abusleme L et al. The subgingival microbiome in health and periodontitis and its relationship with community biomass and inflammation. ISME J. 7, 1016–1025 (2013).
This study documents alterations in subgingival microbial communities that underpin the development of periodontitis and describes the relationship between clinical inflammation and the disease-associated microbiome.
-
-
-
Griffen AL et al. Distinct and complex bacterial profiles in human periodontitis and health revealed by 16S pyrosequencing. ISME J. 6, 1176–1185 (2012).
This landmark study establishes the complexity of the periodontal microbial community and the demarcation between health and disease.
-
-
- Rosan B & Lamont RJ Dental plaque formation. Microbes Infect. 2, 1599–1607 (2000). - PubMed
-
-
Dewhirst FE et al. The human oral microbiome. J. Bacteriol 192, 5002–5017 (2010).
References 4 and 5 are the basis of our current understanding of the diversity of the oral microbiome.
-
Publication types
MeSH terms
Grants and funding
- R37 DE026152/DE/NIDCR NIH HHS/United States
- R01 DE011111/DE/NIDCR NIH HHS/United States
- R01 DE015254/DE/NIDCR NIH HHS/United States
- R01 DE023193/DE/NIDCR NIH HHS/United States
- R01 DE025220/DE/NIDCR NIH HHS/United States
- R01 DE018023/DE/NIDCR NIH HHS/United States
- R01 DE025848/DE/NIDCR NIH HHS/United States
- R01 DE012505/DE/NIDCR NIH HHS/United States
- P01 AI068730/AI/NIAID NIH HHS/United States
- R01 DE024716/DE/NIDCR NIH HHS/United States
- R01 DE017921/DE/NIDCR NIH HHS/United States
- R01 DE024153/DE/NIDCR NIH HHS/United States
LinkOut - more resources
Full Text Sources

