Long-term efficacy and duration of action of dexamethasone implant, in vitrectomised and non-vitrectomised eyes with persistent diabetic macular oedema
- PMID: 30302004
- PMCID: PMC6460697
- DOI: 10.1038/s41433-018-0219-8
Long-term efficacy and duration of action of dexamethasone implant, in vitrectomised and non-vitrectomised eyes with persistent diabetic macular oedema
Abstract
Purpose: To evaluate the efficacy and duration of action of an intravitreal (dexamethasone (Ozurdex)) implant in vitrectomised and non-vitrectomised eyes with persistent diabetic macular oedema (DMO).
Methods: We retrospectively analysed the records for 18 eyes that had or had not been vitrectomised but required an intravitreal dexamethasone implant for DMO after a poor response to anti-vascular endothelial growth factor. Optical coherence tomography and visual acuity (VA) examinations were performed before and 1, 3 and 6 months after implantation. The six months following implantation constituted one treatment round; up to three rounds were studied.
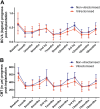
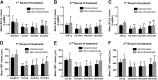
Results: Ten of 18 eyes had undergone vitrectomy. Best-corrected visual acuity (BCVA) and central macular thickness (CMT) were significantly improved by months 1-3 after implantation of the Ozurdex device in all rounds of treatment. The BCVA and CMT deteriorated gradually after month 3 through to month 6 post implantation. There were no statistically significant differences between the vitrectomised and non-vitrectomised groups at any time point. When the implantation interval was <6 weeks from the end of each treatment round, the improvement in BCVA and CMT was obvious even after 18 months of treatment.
Conclusions: Vitrectomy did not have a negative effect on the duration of action or efficacy of the Ozurdex implant in patients with persistent DMO. The implant started working from the first month after implantation regardless of whether vitrectomy had or had not been performed. The maximum functional and anatomic improvement was achieved in the first 3 months post implantation in all treatment rounds.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
Similar articles
-
Effectiveness of Intravitreal Dexamethasone Implant Treatment for Diabetic Macular Edema in Vitrectomized Eyes.Turk J Ophthalmol. 2019 Dec 31;49(6):323-327. doi: 10.4274/tjo.galenos.2019.95226. Turk J Ophthalmol. 2019. PMID: 31893587 Free PMC article.
-
Dexamethasone intravitreal implant in vitrectomized versus nonvitrectomized eyes for treatment of patients with persistent diabetic macular edema.J Ocul Pharmacol Ther. 2014 Nov;30(9):709-16. doi: 10.1089/jop.2014.0010. Epub 2014 Sep 26. J Ocul Pharmacol Ther. 2014. PMID: 25259834
-
Intravitreal dexamethasone implant Ozurdex® in naïve and refractory patients with different subtypes of diabetic macular edema.BMC Ophthalmol. 2019 Jan 11;19(1):15. doi: 10.1186/s12886-018-1022-9. BMC Ophthalmol. 2019. PMID: 30634940 Free PMC article.
-
Fluocinolone acetonide vitreous insert for chronic diabetic macular oedema: a systematic review with meta-analysis of real-world experience.Sci Rep. 2021 Feb 26;11(1):4800. doi: 10.1038/s41598-021-84362-y. Sci Rep. 2021. PMID: 33637841 Free PMC article.
-
Perspective on the role of Ozurdex (dexamethasone intravitreal implant) in the management of diabetic macular oedema.Ther Adv Chronic Dis. 2015 Sep;6(5):234-45. doi: 10.1177/2040622315590319. Ther Adv Chronic Dis. 2015. PMID: 26336592 Free PMC article. Review.
Cited by
-
Initial Ten Years of Experience with the Intravitreal Dexamethasone Implant: A Retrospective Chart Review.Clin Ophthalmol. 2020 Oct 7;14:3097-3108. doi: 10.2147/OPTH.S264559. eCollection 2020. Clin Ophthalmol. 2020. PMID: 33116361 Free PMC article.
-
Efficacy and safety of the dexamethasone implant in vitrectomized and nonvitrectomized eyes with diabetic macular edema: A systematic review and meta-analysis.Front Pharmacol. 2022 Dec 1;13:1029584. doi: 10.3389/fphar.2022.1029584. eCollection 2022. Front Pharmacol. 2022. PMID: 36532786 Free PMC article.
-
The effect of pars plana vitrectomy with internal limiting membrane peeling on the durability of the intravitreal dexamethasone implant in the treatment of diabetic macular edema.Am J Ophthalmol Case Rep. 2022 Feb 17;26:101401. doi: 10.1016/j.ajoc.2022.101401. eCollection 2022 Jun. Am J Ophthalmol Case Rep. 2022. PMID: 35243151 Free PMC article.
-
Vitrectomized vs non-vitrectomized eyes in DEX implant treatment for DMO-Is there any difference? the VITDEX study.Eye (Lond). 2023 Feb;37(2):280-284. doi: 10.1038/s41433-022-01931-9. Epub 2022 Jan 18. Eye (Lond). 2023. PMID: 35043004 Free PMC article.
-
Comparison of Intravitreal Dexamethasone Implant and Ranibizumab in Vitrectomized Eyes with Diabetic Macular Edema.J Ophthalmol. 2021 Sep 10;2021:8882539. doi: 10.1155/2021/8882539. eCollection 2021. J Ophthalmol. 2021. PMID: 34540287 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

