Tracking Tumor Colonization in Xenograft Mouse Models Using Accelerator Mass Spectrometry
- PMID: 30302019
- PMCID: PMC6178347
- DOI: 10.1038/s41598-018-33368-0
Tracking Tumor Colonization in Xenograft Mouse Models Using Accelerator Mass Spectrometry
Abstract
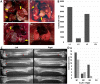
Here we introduce an Accelerator Mass Spectrometry (AMS)-based high precision method for quantifying the number of cancer cells that initiate metastatic tumors, in xenograft mice. Quantification of 14C per cell prior to injection into animals, and quantification of 14C in whole organs allows us to extrapolate the number of cancer cells available to initiate metastatic tumors. The 14C labeling was optimized such that 1 cancer cell was detected among 1 million normal cells. We show that ~1-5% of human cancer cells injected into immunodeficient mice form subcutaneous tumors, and even fewer cells initiate metastatic tumors. Comparisons of metastatic site colonization between a highly metastatic (PC3) and a non-metastatic (LnCap) cell line showed that PC3 cells colonize target tissues in greater quantities at 2 weeks post-delivery, and by 12 weeks post-delivery no 14C was detected in LnCap xenografts, suggesting that all metastatic cells were cleared. The 14C-signal correlated with the presence and the severity of metastatic tumors. AMS measurements of 14C-labeled cells provides a highly-sensitive, quantitative assay to experimentally evaluate metastasis and colonization of target tissues in xenograft mouse models. This approach can potentially be used to evaluate tumor aggressiveness and assist in making informed decisions regarding treatment.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Klerk CP, et al. Validity of bioluminescence measurements for noninvasive in vivo imaging of tumor load in small animals. BioTechniques. 2007;43(7–13):30. - PubMed

