Transformation of amorphous calcium phosphate to bone-like apatite
- PMID: 30302020
- PMCID: PMC6177403
- DOI: 10.1038/s41467-018-06570-x
Transformation of amorphous calcium phosphate to bone-like apatite
Abstract
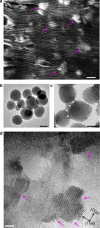
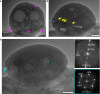
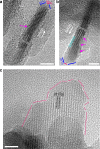
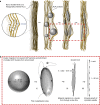
Mineralisation of calcium phosphates in bone has been proposed to proceed via an initial amorphous precursor phase which transforms into nanocrystalline, carbonated hydroxyapatite. While calcium phosphates have been under intense investigation, the exact steps during the crystallisation of spherical amorphous particles to platelet-like bone apatite are unclear. Herein, we demonstrate a detailed transformation mechanism of amorphous calcium phosphate spherical particles to apatite platelet-like crystals, within the confined nanodomains of a bone-inspired nanocomposite. The transformation is initiated under the presence of humidity, where nanocrystalline areas are formed and crystallisation advances via migration of nanometre sized clusters by forming steps at the growth front. We propose that such transformation is a possible crystallisation mechanism and is characteristic of calcium phosphates from a thermodynamic perspective and might be unrelated to the environment. Our observations provide insight into a crucial but unclear stage in bone mineralisation, the origins of the nanostructured, platelet-like bone apatite crystals.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Dunlop JWC, Fratzl P. Biological composites. Annu. Rev. Mater. Sci. 2010;40:1–24. doi: 10.1146/annurev-matsci-070909-104421. - DOI
-
- Fratzl P, Gupta HS, Paschalis EP, Roschger P. Structure and mechanical quality of the collagen–mineral nano-composite in bone. J. Mater. Chem. 2004;14:2115–2123. doi: 10.1039/B402005G. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

