Impact of Direct Acting Antiviral Drugs in Treatment Naïve HCV Cirrhosis on Fibrosis and Severity of Liver Disease: A Real Life Experience from a Tertiary Care Center of North India
- PMID: 30302040
- PMCID: PMC6175719
- DOI: 10.1016/j.jceh.2017.11.011
Impact of Direct Acting Antiviral Drugs in Treatment Naïve HCV Cirrhosis on Fibrosis and Severity of Liver Disease: A Real Life Experience from a Tertiary Care Center of North India
Abstract
Background/aims: Treatment of chronic hepatitis C infection with direct-acting antiviral (DAA) drugs has been highly effective, but data regarding benefit in advanced liver disease is relatively scarce in Indian patients. The aim of this study was to determine the effects of DAA in patients with HCV related cirrhosis (compensated/decompensated) who achieved sustained virological response post-therapy at 12 weeks (SVR12).
Methods: Sixty-three patients with HCV related cirrhosis treated with sofosbuvir based regimen were evaluated. Data regarding baseline demographics, the severity of liver disease and treatment regimen were collected. The primary end point was to evaluate the effect of treatment (SVR12) on the severity of liver disease with the secondary end point being to observe for any adverse events related to treatment.
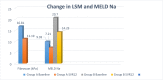
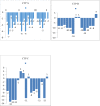
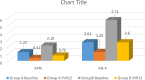
Results: Treatment naïve patients with HCV cirrhosis either due to genotype 1 or genotype 3 were divided into two groups: group A (compensated cirrhosis), group B (decompensated cirrhosis). SVR12 in group A was 91.66% (33/37) and in group, B was 73.17% (30/41). Baseline mean liver stiffness measurement (LSM) in group A was 16.81 ± 3.57 kPa which decreased to 11.19 ± 1.75 kPa at SVR12 (P-value <0.0001). Baseline mean APRI and FIB-4 score in group A were 1.228 ± 0.499 and 2.61 ± 1.06 and in group B were 2.156 ± 1.10 and 5.71 ± 2.06 respectively which decrease to 0.415 ± 0.115 and 1.25 ± 0.46 in group A, to 0.759 ± 0.275 and 2.60 ± 1.12 in group B following SVR12 (P value <0.0001). Mean MELD-Na improved from baseline 9.93 ± 2.04, 20.70 ± 4.52 to 7.21 ± 0.92, 14.23 ± 4.51 respectively in group A and B at SVR12 (P-value <0.0001). Child-Turcotte-Pugh score improved by 1 in 27.27% (9/33) and ≥2 in 76.67% (23/30) of patients in group A and group B respectively.
Conclusion: There was a significant improvement in severity of liver disease as depicted by the decrease in LSM and other noninvasive marker of fibrosis in patients who achieved SVR12 on DAA therapy.
Keywords: APRI, AST to Platelet Ratio Index; CTP, Child-Turcotte-Pugh; CTP, Child–Turcotte–Pugh; FIB-4, fibrosis-4; MELD Na, Model for End-Stage Liver Disease sodium; TE, transient elastography.
Figures
References
-
- Mohd Hanafiah K., Groeger J., Flaxman A.D., Wiersma S.T. Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology. 2013;57:1333–1342. - PubMed
-
- Narahari S., Juwle A., Basak S., Saranath D. Prevalence and geographic distribution of hepatitis C virus genotypes in Indian patient cohort. Infect Genet Evol. 2009;9:643–645. - PubMed
-
- Seeff L.B. Natural history of chronic hepatitis C. Hepatology. 2002;36(suppl 1):S35–S46. - PubMed
-
- Fattovich G., Giustina G., Degos F. Morbidity and mortality in compensated cirrhosis type C: a retrospective follow-up study of 384 patients. Gastroenterology. 1997;112:463–472. - PubMed
-
- Van der Meer A.J., Veldt B.J., Feld J.J. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. JAMA. 2012;308:2584–2593. - PubMed
LinkOut - more resources
Full Text Sources