UK clinical experience up to 52 weeks with linaclotide for irritable bowel syndrome with constipation
- PMID: 30302125
- PMCID: PMC6170957
- DOI: 10.1177/1756284818798791
UK clinical experience up to 52 weeks with linaclotide for irritable bowel syndrome with constipation
Abstract
Background: Linaclotide, a guanylate cyclase C agonist, has been shown in clinical trials to improve symptoms of irritable bowel syndrome with constipation (IBS-C). Here we report data from a real-world study of linaclotide in the UK.
Methods: This 1-year, multicentre, prospective, observational study in the UK enrolled patients aged 18 years and over initiating linaclotide for IBS-C. The primary assessment was change from baseline in IBS Symptom Severity Scale (IBS-SSS) score at 12 weeks, assessed in patients with paired baseline and 12-week data. Change from baseline in IBS-SSS score at 52 weeks was a secondary assessment. Adverse events were recorded.
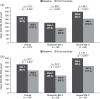
Results: In total, 202 patients were enrolled: 185 (91.6%) were female, median age was 44.9 years (range 18.1-77.2) and 84 (41.6%) reported baseline laxative use. Mean (standard deviation) baseline IBS-SSS score was 339 (92), with most patients (n = 129; 66.8%) classified as having severe disease (score ⩾300). In patients with paired data, there was a significant mean (95% confidence interval) decrease in IBS-SSS score from baseline to 12 weeks [-77.0 (-96.3, -57.7); p < 0.001; n = 124] and baseline to 52 weeks [-70.7 (-95.0, -46.5); p < 0.001; n = 76]. Overall, 174 adverse events were reported in 77 (38.1%) patients, most commonly diarrhoea (n = 54; 26.7%), abdominal pain (n = 21; 10.4%) and abdominal distension (n = 13; 6.4%).
Conclusion: Linaclotide significantly improved IBS-SSS score at 12 and 52 weeks. These results provide insights into outcomes with linaclotide treatment over 1 year in patients with IBS-C in real-world clinical practice.
Keywords: irritable bowel syndrome with constipation; linaclotide; observational study.
Conflict of interest statement
Conflict of interest statement: Financial arrangements of the authors with companies whose products may be related to the present report are listed below, as declared by the authors. Y. Yiannakou has received funding from Accretio, Allergan plc, Almirall, Coloplast, Enteromed, Kyowa Kirin, Medtronic and Shire. A. Agrawal has no conflict of interest to declare. P.B. Allen has participated in advisory boards for Allergan plc. N. Arebi has received funding from Allergan plc and Almirall. S.R. Brown has received conference funding from Allergan plc. M.P. Eugenicos has participated in advisory boards for Allergan plc, Almirall, Dr Falk Pharma, Kyowa Kirin and Shire. A.D. Farmer has participated in advisory boards or given lectures for Allergan plc, Almirall, Kyowa Kirin and Shire. S. McLain-Smith is an employee of pH Associates Ltd and has carried out data analysis and provided medical writing support. J. McLaughlin received an award to act as a keynote speaker at a meeting organised by Almirall. D.S. Sanders has participated in advisory boards or given lectures for Allergan plc and Almirall. D. Lawrance is an employee of Allergan plc. A. Emmanuel has participated in advisory boards for Allergan plc, Almirall, Shire and Takeda.
Figures




References
-
- Chey WD, Kurlander J, Eswaran S. Irritable bowel syndrome: a clinical review. JAMA 2015; 313: 949–958. - PubMed
-
- Lacy BE, Mearin F, Chang L, et al. Bowel disorders. Gastroenterology 2016; 150: 1393–1407. - PubMed
-
- Drossman DA, Camilleri M, Mayer EA, et al. AGA technical review on irritable bowel syndrome. Gastroenterology 2002; 123: 2108–2131. - PubMed
-
- Hungin APS, Whorwell PJ, Tack J, et al. The prevalence, patterns and impact of irritable bowel syndrome: an international survey of 40 000 subjects. Aliment Pharmacol Ther 2003; 17: 643–650. - PubMed

