Five-year direct costs of acute lymphoblastic leukemia pediatric patients undergoing allogeneic stem cell transplant
- PMID: 30302205
- PMCID: PMC6171974
- DOI: 10.2217/ijh-2016-0001
Five-year direct costs of acute lymphoblastic leukemia pediatric patients undergoing allogeneic stem cell transplant
Abstract
Aim: To assess the 5-year healthcare resource utilization (HRU) and direct payer costs following allogeneic hematopoietic stem cell transplants (HSCTs) in acute lymphoblastic leukemia pediatric patients using data from two large US administrative databases.
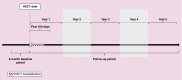
Patients & methods: Among the 209 patients with acute lymphoblastic leukemia, HRU and costs were described over the up to 5 years after the HSCT.
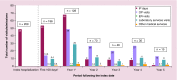
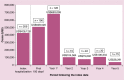
Results: HRU and costs following the HSCTs were substantial. The highest average costs and most intensive HRU were observed within the first year following the HSCTs (49 outpatient visits; 29 laboratory service visits; 68 inpatient days), with a first year cost of US$683,099 and substantial costs over the following years.
Conclusion: HRU and direct costs associated with allogeneic HSCTs are substantial.
Keywords: acute lymphoblastic leukemia; allogeneic stem cell transplantation; direct costs; economic burden; healthcare resource utilization; pediatric patients.
Conflict of interest statement
Financial & competing interests disclosure Funding for this research was provided by Novartis Pharmaceuticals Corporation. RT Maziarz is an employee of the Oregon Health & Science University who provided and received payment for consultant services to Novartis Pharmaceuticals Corporation. This potential conflict of interest has been reviewed and managed by OHSU. SK Thomas and L Chen are employees of Novartis Pharmaceuticals Corporation and own stock/stock options. G Gauthier, J Heroux, M Zhdanava, A Guérin and EQ Wu are employees of Analysis Group Inc., which has received consultancy fees from Novartis Pharmaceuticals Corporation for this project. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. Writing assistance was utilized in the production of this manuscript. Shelley Batts, an employee of Analysis Group Inc., aided with formatting and editing this manuscript. Analysis Group Inc., has received consultancy fees from Novartis Pharmaceuticals Corporation for this project.
Figures



References
-
- Pasquini M, Zhu X. Current use and outcome of hematopoietic stem cell transplantation. CIBMTR Summary Slides. 2011. www.cibmtr.org
-
• The annual report of the Center for International Blood and Marrow Transplant Research on worldwide practices and general survival outcomes after hematopoietic cell transplantation – contains data on the number of hematopoietic stem cell transplants (HSCT) performed in the USA, describes information related to practices and outcomes post-transplantation.
-
- Oliansky DM, Camitta B, Gaynon P, et al. Role of cytotoxic therapy with hematopoietic stem cell transplantation in the treatment of pediatric acute lymphoblastic leukemia: update of the 2005 evidence-based review. Biol. Blood Marrow Transplant. 2012;18(4):505–522. - PubMed
-
- Oliansky DM, Rizzo JD, Aplan PD, et al. The role of cytotoxic therapy with hematopoietic stem cell transplantation in the therapy of acute myeloid leukemia in children: an evidence-based review. Biol. Blood Marrow Transplant. 2007;13(1):1–25. - PubMed
LinkOut - more resources
Full Text Sources
