Abnormal gametogenesis induced by p53 deficiency promotes tumor progression and drug resistance
- PMID: 30302273
- PMCID: PMC6167385
- DOI: 10.1038/s41421-018-0054-x
Abnormal gametogenesis induced by p53 deficiency promotes tumor progression and drug resistance
Abstract
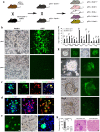
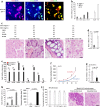
The century-old embryonal/gametogenesis hypothesis of tumors could link diverse tumors' malignant features together likely representing the real "stemness" of tumors. However, the genetic evidence to validate abnormal gametogenesis in tumors remains lacking. Here we show that p53 deficiency elicits abnormal gametogenesis from primordial germ cell-like stage to late oocyte-like stage and subsequent parthenogenetic activation. The similar upregulation of abnormal gametogenesis by p53 deficiency is observed both in p53-/- mouse model and cultured cancer cells. Notably, germ cell-like cells isolated from distinct tumors from p53-/- mice and cancer cell lines display potent tumorigenicity potential. Abnormal oogenesis induced by p53 deficiency and then spontaneous parthenogenetic activation endow tumors with imitated embryonic development, life cycle, and therapeutic resistance. Our study establishes the genetic evidence to support embryonal/gametogenesis theory of tumors and reveals a pivotal role of p53 in restricting abnormal gametogenesis that may represent a novel aspect for p53's tumor suppression.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures




References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

