AS03-adjuvanted H5N1 vaccine promotes antibody diversity and affinity maturation, NAI titers, cross-clade H5N1 neutralization, but not H1N1 cross-subtype neutralization
- PMID: 30302282
- PMCID: PMC6167326
- DOI: 10.1038/s41541-018-0076-2
AS03-adjuvanted H5N1 vaccine promotes antibody diversity and affinity maturation, NAI titers, cross-clade H5N1 neutralization, but not H1N1 cross-subtype neutralization
Abstract
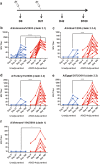
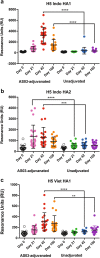
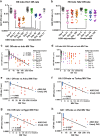
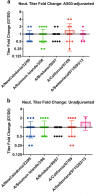
Immune responses to inactivated vaccines against avian influenza are poor due in part to lack of immune memory. Adjuvants significantly increased virus neutralizing titers. We performed comprehensive analyses of polyclonal antibody responses following FDA-approved adjuvanted H5N1-A/Indonesia vaccine, administered in presence or absence of AS03. Using Whole Genome Fragment Phage Display Libraries, we observed that AS03 induced antibody epitope diversity to viral hemagglutinin (HA) and neuraminidase compared with unadjuvanted vaccine. Furthermore, AS03 promoted significant antibody affinity maturation to properly folded H5-HA1 (but not to HA2) domain, which correlated with neutralization titers against both vaccine and heterologous H5N1 strains. However, no increase in heterosubtypic cross-neutralization of Group1-H1N1 seasonal strains was observed. AS03-H5N1 vaccine also induced higher neuraminidase inhibition antibody titers. This study provides insight into the differential impacts of AS03 adjuvant on H5N1 vaccine-induced antibody responses that may help optimize vaccine platforms for future vaccines with improved protection against seasonal and pandemic influenza strains.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

