System immunology-based identification of blood transcriptional modules correlating to antibody responses in sheep
- PMID: 30302283
- PMCID: PMC6170373
- DOI: 10.1038/s41541-018-0078-0
System immunology-based identification of blood transcriptional modules correlating to antibody responses in sheep
Erratum in
-
Erratum: Author Correction: System immunology-based identification of blood transcriptional modules correlating to antibody responses in sheep.NPJ Vaccines. 2019 Feb 19;4:10. doi: 10.1038/s41541-019-0100-1. eCollection 2019. NPJ Vaccines. 2019. PMID: 30792904 Free PMC article.
Abstract
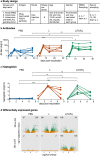
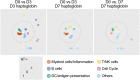
Inactivated vaccines lack immunogenicity and therefore require potent adjuvants. To understand the in vivo effects of adjuvants, we used a system immunology-based analysis of ovine blood transcriptional modules (BTMs) to dissect innate immune responses relating to either antibody or haptoglobin levels. Using inactivated foot-and-mouth disease virus as an antigen, we compared non-adjuvanted to liposomal-formulated vaccines complemented or not with TLR4 and TLR7 ligands. Early after vaccination, BTM relating to myeloid cells, innate immune responses, dendritic cells, and antigen presentation correlated positively, whereas BTM relating to T and natural killer cells, as well as cell cycle correlated negatively with antibody responses. Interestingly, similar BTM also correlated with haptoglobin, but in a reversed manner, indicating that acute systemic inflammation is not beneficial for early antibody responses. Analysis of vaccine-dependent BTM modulation showed that liposomal formulations induced similar responses to those correlating to antibody levels. Surprisingly, the addition of the TLR ligands appeared to reduce early immunological perturbations and mediated anti-inflammatory effects, despite promoting antibody responses. When pre-vaccination BTM were analyzed, we found that high vaccine responders expressed higher levels of many BTM relating to cell cycle, antigen-presenting cells, and innate responses as compared with low responders. In conclusion, we have transferred human BTM to sheep and identified early vaccine-induced responses associated with antibody levels or unwanted inflammation in a heterogeneous and small group of animals. Such readouts are applicable to other veterinary species and very useful to identify efficient vaccine adjuvants, their mechanism of action, and factors related to low responders.
Conflict of interest statement
The authors declare no competing interests.
Figures