Malaria vaccine candidate based on Duffy-binding protein elicits strain transcending functional antibodies in a Phase I trial
- PMID: 30302285
- PMCID: PMC6162314
- DOI: 10.1038/s41541-018-0083-3
Malaria vaccine candidate based on Duffy-binding protein elicits strain transcending functional antibodies in a Phase I trial
Abstract
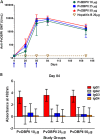
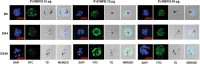
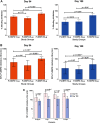
Reticulocyte invasion by Plasmodium vivax requires interaction of the Duffy-binding protein (PvDBP) with host Duffy antigen receptor for chemokines (DARCs). The binding domain of PvDBP maps to a cysteine-rich region referred to as region II (PvDBPII). Blocking this interaction offers a potential path to prevent P. vivax blood-stage growth and P. vivax malaria. This forms the rationale for development of a vaccine based on PvDBPII. Here we report results of a Phase I randomized trial to evaluate the safety and immunogenicity of recombinant PvDBPII formulated with glucopyranosyl lipid adjuvant-stable emulsion (GLA-SE). Thirty-six malaria-naive, healthy Indian male subjects aged 18-45 years were assigned into three cohorts corresponding to doses of 10, 25 and 50 µg of PvDBPII formulated with 5 µg of GLA-SE. Each cohort included nine PvDBPII/GLA-SE vaccinees and three hepatitis B control vaccine recipients. Each subject received the assigned vaccine intramuscularly on days 0, 28 and 56, and was followed up till day 180. No serious AE was reported and PvDBPII/GLA-SE was well-tolerated and safe. Analysis by ELISA showed that all three doses of PvDBPII elicited antigen-specific binding-inhibitory antibodies. The 50 µg dose elicited antibodies against PvDBPII that had the highest binding-inhibitory titres and were most persistent. Importantly, the antibody responses were strain transcending and blocked receptor binding of diverse PvDBP alleles. These results support further clinical development of PvDBPII/GLA-SE to evaluate efficacy against sporozoite or blood-stage challenge in controlled human malaria infection (CHMI) models and against natural P. vivax challenge in malaria endemic areas.
Conflict of interest statement
D.C.K. is an inventor on patents that relate to DNA segments encoding the Duffy receptor of Plasmodium parasite, including recombinantly produced Duffy receptor. C.E.C. is an inventor on patents that relate to binding domains of erythrocyte binding proteins of Plasmodium parasites including PvDBP. S.R. and D.C. are inventors on patents covering the adjuvant GLA-SE. None of the other authors have any competing interests.
Figures
References
-
- WHO. Global Malaria Programme: World Malaria Report 2017. Geneva: World Health Organization; 2017.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

