Crystallography and Its Impact on Carbonic Anhydrase Research
- PMID: 30302289
- PMCID: PMC6158936
- DOI: 10.1155/2018/9419521
Crystallography and Its Impact on Carbonic Anhydrase Research
Abstract
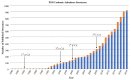
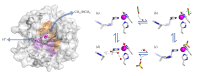
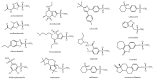
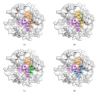
X-ray and neutron crystallography are powerful techniques utilized to study the structures of biomolecules. Visualization of enzymes in complex with substrate/product and the capture of intermediate states can be related to activity to facilitate understanding of the catalytic mechanism. Subsequent analysis of small molecule binding within the enzyme active site provides insight into mechanisms of inhibition, supporting the design of novel inhibitors using a structure-guided approach. The first X-ray crystal structures were determined for small, ubiquitous enzymes such as carbonic anhydrase (CA). CAs are a family of zinc metalloenzymes that catalyze the hydration of CO2, producing HCO3 - and a proton. The CA structure and ping-pong mechanism have been extensively studied and are well understood. Though the function of CA plays an important role in a variety of physiological functions, CA has also been associated with diseases such as glaucoma, edema, epilepsy, obesity, and cancer and is therefore recognized as a drug target. In this review, a brief history of crystallography and its impact on CA research is discussed.
Figures


References
-
- Friedrich W., Knipping P., von Laue M. Interference Phenomena with rontgen rays. Annals of Physics. 1913;41:971–988.
-
- Bragg W. L. The diffraction of short electromagnetic waves by a crystal. Mathematical Proceedings of the Cambridge Philosophical Society. 1913;17:43–57.
-
- Bragg W. L. The structure of some crystals as indicated by their diffraction of X-rays. Proceedings of the Royal Society A Mathematical, Physical and Engineering Sciences. 1913;89(610):248–277. doi: 10.1098/rspa.1913.0083. - DOI
-
- Bragg W. H., Bragg W. L. The reflection of X-rays by crystals. Proceedings of the Royal Society A Mathematical, Physical and Engineering Sciences. 1913;88(605):428–438. doi: 10.1098/rspa.1913.0040. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

