Microbial esterases and ester prodrugs: An unlikely marriage for combating antibiotic resistance
- PMID: 30302779
- PMCID: PMC6377847
- DOI: 10.1002/ddr.21468
Microbial esterases and ester prodrugs: An unlikely marriage for combating antibiotic resistance
Abstract
The rise of antibiotic resistance necessitates the search for new platforms for drug development. Prodrugs are common tools for overcoming drawbacks typically associated with drug formulation and delivery, with ester prodrugs providing a classic strategy for masking polar alcohol and carboxylic acid functionalities and improving cell permeability. Ester prodrugs are normally designed to have simple ester groups, as they are expected to be cleaved and reactivated by a wide spectrum of cellular esterases. However, a number of pathogenic and commensal microbial esterases have been found to possess significant substrate specificity and can play an unexpected role in drug metabolism. Ester protection can also introduce antimicrobial properties into previously nontoxic drugs through alterations in cell permeability or solubility. Finally, mutation to microbial esterases is a novel mechanism for the development of antibiotic resistance. In this review, we highlight the important pathogenic and xenobiotic functions of microbial esterases and discuss the development and application of ester prodrugs for targeting microbial infections and combating antibiotic resistance. Esterases are often overlooked as therapeutic targets. Yet, with the growing need to develop new antibiotics, a thorough understanding of the specificity and function of microbial esterases and their combined action with ester prodrug antibiotics will support the design of future therapeutics.
Keywords: Mycobacterium tuberculosis; antibiotic resistance; drug delivery; drug design; esterases; prodrugs.
© 2018 Wiley Periodicals, Inc.
Figures
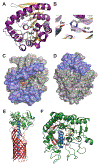
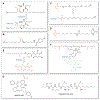


References
-
- Abet V, Filace F, Recio J, Alvarez-Builla J, Burgos C. 2017. Prodrug approach: An overview of recent cases. Eur J Med Chem 127:810–827. - PubMed
-
- Alanis AJ. 2005. Resistance to antibiotics: are we in the post-antibiotic era? Arch Med Res 36(6):697–705. - PubMed
-
- Arias LR, Yang L. 2009. Inactivation of bacterial pathogens by carbon nanotubes in suspensions. Langmuir 25(5):3003–3012. - PubMed
-
- Assali M, Joulani M, Awwad R, Assad M, Almasri M, Kittana N, Zaid AN. 2016. Facile synthesis of ciprofloxacin prodrug analogues to improve its water solubility and antibacterial activity. Chemistry select 1(6):1132–1135.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

