Concise Review: The Current State of Human In Vitro Cardiac Disease Modeling: A Focus on Gene Editing and Tissue Engineering
- PMID: 30302938
- PMCID: PMC6312446
- DOI: 10.1002/sctm.18-0052
Concise Review: The Current State of Human In Vitro Cardiac Disease Modeling: A Focus on Gene Editing and Tissue Engineering
Abstract
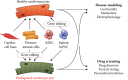
Until recently, in vivo and ex vivo experiments were the only means to determine factors and pathways involved in disease pathophysiology. After the generation of characterized human embryonic stem cell lines, human diseases could readily be studied in an extensively controllable setting. The introduction of human-induced pluripotent stem cells, a decade ago, allowed the investigation of hereditary diseases in vitro. In the field of cardiology, diseases linked to known genes have successfully been studied, revealing novel disease mechanisms. The direct effects of various mutations leading to hypertrophic cardiomyopathy, dilated cardiomyopathy, arrythmogenic cardiomyopathy, or left ventricular noncompaction cardiomyopathy are discovered as a result of in vitro disease modeling. Researchers are currently applying more advanced techniques to unravel more complex phenotypes, resulting in state-of-the-art models that better mimic in vivo physiology. The continued improvement of tissue engineering techniques and new insights into epigenetics resulted in more reliable and feasible platforms for disease modeling and the development of novel therapeutic strategies. The introduction of CRISPR-Cas9 gene editing granted the ability to model diseases in vitro independent of induced pluripotent stem cells. In addition to highlighting recent developments in the field of human in vitro cardiomyopathy modeling, this review also aims to emphasize limitations that remain to be addressed; including residual somatic epigenetic signatures induced pluripotent stem cells, and modeling diseases with unknown genetic causes. Stem Cells Translational Medicine 2019;8:66-74.
Keywords: Cardiac disease; Heart failure; In vitro disease models; Stem cells.
© 2018 The Authors. Stem Cells Translational Medicine published by Wiley Periodicals, Inc. on behalf of AlphaMed Press.
Figures

References
-
- Benjamin EJ, Virani SS, Callaway CW et al. Heart disease and stroke statistics—2018 update: A report from the American heart association. Circulation 2018;137:e67–e492. - PubMed
-
- Maron BJ, Maron MS. Hypertrophic cardiomyopathy. Lancet 2013;381:242–255. - PubMed
-
- Weintraub RG, Semsarian C, Macdonald P. Dilated cardiomyopathy. Lancet 2017;390:400–414. - PubMed
-
- Rampazzo A, Nava A, Danieli GA et al. The gene for arrhythmogenic right ventricular cardiomyopathy maps to chromosome 14q23–q24. Hum Mol Genet 1994;3:959–962. - PubMed
-
- Jefferies JL, Towbin JA. Dilated cardiomyopathy. Lancet 2010;375:752–762. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

