Oxidative stress and inflammation in a spectrum of epileptogenic cortical malformations: molecular insights into their interdependence
- PMID: 30303592
- PMCID: PMC8028690
- DOI: 10.1111/bpa.12661
Oxidative stress and inflammation in a spectrum of epileptogenic cortical malformations: molecular insights into their interdependence
Abstract
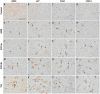
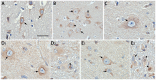
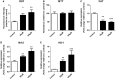
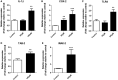
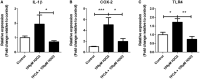
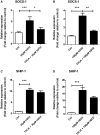
Oxidative stress (OS) occurs in brains of patients with epilepsy and coincides with brain inflammation, and both phenomena contribute to seizure generation in animal models. We investigated whether expression of OS and brain inflammation markers co-occurred also in resected brain tissue of patients with epileptogenic cortical malformations: hemimegalencephaly (HME), focal cortical dysplasia (FCD) and cortical tubers in tuberous sclerosis complex (TSC). Moreover, we studied molecular mechanisms linking OS and inflammation in an in vitro model of neuronal function. Untangling interdependency and underlying molecular mechanisms might pose new therapeutic strategies for treating patients with drug-resistant epilepsy of different etiologies. Immunohistochemistry was performed for specific OS markers xCT and iNOS and brain inflammation markers TLR4, COX-2 and NF-κB in cortical tissue derived from patients with HME, FCD IIa, IIb and TSC. Additionally, we studied gene expression of these markers using the human neuronal cell line SH-SY5Y in which OS was induced using H2 O2 . OS markers were higher in dysmorphic neurons and balloon/giant cells in cortex of patients with FCD IIb or TSC. Expression of OS markers was positively correlated to expression of brain inflammation markers. In vitro, 100 µM, but not 50 µM, of H2 O2 increased expression of TLR4, IL-1β and COX-2. We found that NF-κB signaling was activated only upon stimulation with 100 µM H2 O2 leading to upregulation of TLR4 signaling and IL-1β. The NF-κB inhibitor TPCA-1 completely reversed this effect. Our results show that OS positively correlates with neuroinflammation and is particularly evident in brain tissue of patients with FCD IIb and TSC. In vitro, NF-κB is involved in the switch to an inflammatory state after OS. We propose that the extent of OS can predict the neuroinflammatory state of the brain. Additionally, antioxidant treatments may prevent the switch to inflammation in neurons thus targeting multiple epileptogenic processes at once.
Keywords: epilepsy; focal cortical dysplasia; hemimegalencephaly; inflammation; oxidative stress; tuberous sclerosis complex.
© 2018 International Society of Neuropathology.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
-
- Aronica E, Boer K, Baybis M, Yu J, Crino P (2007) Co‐expression of cyclin D1 and phosphorylated ribosomal S6 proteins in hemimegalencephaly. Acta Neuropathol 114:287–293. - PubMed
-
- Aronica E, Crino PB (2011) Inflammation in epilepsy: clinical observations. Epilepsia 52(Suppl. 3):26–32. - PubMed
-
- Aronica E, Muhlebner A (2017) Neuropathology of epilepsy. Handb Clin Neurol 145:193–216. - PubMed
-
- Baybis M, Yu J, Lee A, Golden JA, Weiner H, McKhann G 2nd et al (2004) mTOR cascade activation distinguishes tubers from focal cortical dysplasia. Ann Neurol 56:478–487. - PubMed
-
- Ben‐Menachem E, Kyllerman M, Marklund S (2000) Superoxide dismutase and glutathione peroxidase function in progressive myoclonus epilepsies. Epilepsy Res 40:33–39. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Research Materials

