The Difference δ-Cells Make in Glucose Control
- PMID: 30303773
- PMCID: PMC6347098
- DOI: 10.1152/physiol.00029.2018
The Difference δ-Cells Make in Glucose Control
Abstract
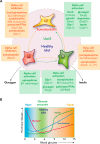
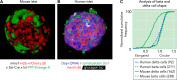
The role of beta and α-cells to glucose control are established, but the physiological role of δ-cells is poorly understood. Delta-cells are ideally positioned within pancreatic islets to modulate insulin and glucagon secretion at their source. We review the evidence for a negative feedback loop between delta and β-cells that determines the blood glucose set point and suggest that local δ-cell-mediated feedback stabilizes glycemic control.
Figures


References
-
- Adriaenssens AE, Svendsen B, Lam BY, Yeo GS, Holst JJ, Reimann F, Gribble FM. Transcriptomic profiling of pancreatic alpha, beta and δ-cell populations identifies δ-cells as a principal target for ghrelin in mouse islets. Diabetologia 59: 2156–2165, 2016. doi:10.1007/s00125-016-4033-1. - DOI - PMC - PubMed
-
- Benner C, van der Meulen T, Cacéres E, Tigyi K, Donaldson CJ, Huising MO. The transcriptional landscape of mouse β-cells compared to human β-cells reveals notable species differences in long non-coding RNA and protein-coding gene expression. BMC Genomics 15: 620, 2014. doi:10.1186/1471-2164-15-620. - DOI - PMC - PubMed

