Somatic Mutations of TSC2 or MTOR Characterize a Morphologically Distinct Subset of Sporadic Renal Cell Carcinoma With Eosinophilic and Vacuolated Cytoplasm
- PMID: 30303819
- PMCID: PMC6686675
- DOI: 10.1097/PAS.0000000000001170
Somatic Mutations of TSC2 or MTOR Characterize a Morphologically Distinct Subset of Sporadic Renal Cell Carcinoma With Eosinophilic and Vacuolated Cytoplasm
Abstract
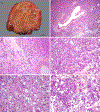
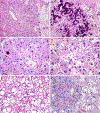
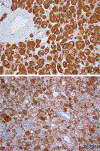
The differential diagnosis of renal cell neoplasms with solid or nested architecture and eosinophilic cytoplasm has become increasingly complex. Despite recent advances in classifying a number of entities exhibiting this morphology, some tumors remain in the unclassified category. Here we describe a morphologically distinct group of sporadic renal cell carcinoma (RCC) with predominantly nested architecture, eosinophilic, and remarkably vacuolated cytoplasm retrospectively identified from a cohort of previously unclassified tumors. We examined the clinicopathologic and immunohistochemical features of these tumors and investigated their mutational and copy number alterations using a targeted next-generation sequencing platform. The study included 7 patients with a mean age of 54 years (range: 40 to 68 y) and a male to female ratio of 3:4. All patients presented with a solitary renal mass and had no prior medical or family history raising concern for syndromic conditions. Tumors were well-circumscribed, unencapsulated, and comprised of nests of eosinophilic cells in a hypocellular and often edematous stroma. Tumor cells had round nuclei with prominent nucleoli and granular cytoplasm with striking vacuolization. Thick-walled vessels and calcifications were also frequently present, whereas increased mitotic activity, necrosis, foamy histiocytes or lymphocytic infiltrates were not identified. All cases were positive for PAX8, had retained expression of SDHB and FH, and exhibited a CK7-/CK20- phenotype. While cathepsin-K was positive in 5 cases, none exhibited immunoreactivity to HMB45 or Melan A, or TFE3 immunostaining. Next-generation sequencing identified somatic inactivating mutations of TSC2 (3/5 tumors tested) or activating mutations of MTOR (2/5) as the primary molecular alterations, consistent with hyperactive mTOR complex 1 signaling which was further demonstrated by phospho-S6 and phospho-4E-BP1 immunostaining. Copy number analysis revealed a loss of chromosome 1 in both cases with MTOR mutation. These tumors represent a novel subset of sporadic RCC characterized by alterations in TSC1-TSC2 complex or the mTOR complex 1 pathway. Recognition of their characteristic morphologic and immunophenotypic features will allow them to be readily identified and separated from the unclassified RCC category.
Conflict of interest statement
Disclosures: The authors have no conflicts of interest to disclose.
Figures


References
-
- Pavlovich CP, Walther MM, Eyler RA, et al. Renal tumors in the Birt-Hogg-Dube syndrome. Am J Surg Pathol. 2002;26:1542–1552. - PubMed
-
- Furuya M, Yao M, Tanaka R, et al. Genetic, epidemiologic and clinicopathologic studies of Japanese Asian patients with Birt-Hogg-Dube syndrome. Clin Genet. 2016;90:403–412. - PubMed
-
- Tickoo SK, Reuter VE, Amin MB, et al. Renal oncocytosis: a morphologic study of fourteen cases. Am J Surg Pathol. 1999;23:1094–1101. - PubMed
-
- Guo J, Tretiakova MS, Troxell ML, et al. Tuberous sclerosis-associated renal cell carcinoma: a clinicopathologic study of 57 separate carcinomas in 18 patients. Am J Surg Pathol. 2014;38:1457–1467. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

