Elagolix Alone or With Add-Back Therapy in Women With Heavy Menstrual Bleeding and Uterine Leiomyomas: A Randomized Controlled Trial
- PMID: 30303923
- PMCID: PMC7963450
- DOI: 10.1097/AOG.0000000000002933
Elagolix Alone or With Add-Back Therapy in Women With Heavy Menstrual Bleeding and Uterine Leiomyomas: A Randomized Controlled Trial
Abstract
Objective: To evaluate elagolix, an oral gonadotropin-releasing hormone receptor antagonist, alone or with add-back therapy, in premenopausal women with heavy menstrual bleeding (greater than 80 mL per month) associated with uterine leiomyomas.
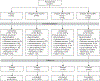
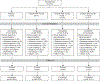
Methods: This double-blind, randomized, placebo-controlled, parallel-group study evaluated efficacy and safety of elagolix in cohorts 1 (300 mg twice daily) and 2 (600 mg daily) with four arms per cohort: placebo, elagolix alone, elagolix with 0.5 mg estradiol/0.1 norethindrone acetate, and elagolix with 1.0 mg estradiol/0.5 mg norethindrone acetate. A sample size of 65 per group was planned to compare elagolix with add-back to placebo on the primary end point: the percentage of women who had less than 80 mL menstrual blood loss and 50% or greater reduction in menstrual blood loss from baseline to the last 28 days of treatment. Safety assessments included changes in bone mineral density.
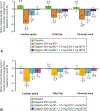
Results: From April 8, 2013, to December 8, 2015, 571 women were enrolled, 567 were randomized and treated (cohort 1=259; cohort 2=308), and 80% and 75% completed treatment, respectively. Participants had a mean±SD age of 43±5 years (cohort 2, 42±5 years), and 70% were black (cohort 2, 74%). Primary end point responder rates in cohort 1 (cohort 2) were 92% (90%) for elagolix alone, 85% (73%) for elagolix with 0.5 mg estradiol/0.1 mg norethindrone acetate, 79% (82%) for elagolix with 1.0 mg estradiol/0.5 mg norethindrone acetate, and 27% (32%) for placebo (all P<.001 vs placebo). Elagolix groups had significant decreases compared with placebo in lumbar spine bone mineral density, which was attenuated by adding 1.0 mg estradiol/0.5 mg norethindrone acetate.
Conclusion: Elagolix with and without add-back significantly reduced menstrual blood loss in women with uterine leiomyomas. Add-back therapy reduced hypoestrogenic effects on bone mineral density.
Clinical trial registration: ClinicalTrials.gov, NCT01817530; EU Clinical Trial Register, 2013-000082-37.
Figures


References
-
- Stewart EA, Laughlin-Tommaso SK, Catherino WH, Lalitkumar S, Gupta D, Vollenhoven B. Uterine fibroids. Nat Rev Dis Primers 2016;2:16043. - PubMed
-
- Baird DD, Dunson DB, Hill MC, Cousins D, Schectman JM. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol 2003;188:100–7. - PubMed
-
- Valladares F, Frías I, Báez D, García C, López FJ, Fraser JD, et al. Characterization of estrogen receptors alpha and beta in uterine leiomyoma cells. Fertil Steril 2006;86:1736–43. - PubMed
-
- Dvorská D, Braný D, Danková Z, Halašová E, Višňovský J. Molecular and clinical attributes of uterine leiomyomas. Tumour Biol 2017;39:1010428317710226. - PubMed
-
- Carr BR, Marshburn PB, Weatherall PT, Bradshaw KD, Breslau NA, Byrd W, et al. An evaluation of the effect of gonadotropin-releasing hormone analogs and medroxyprogesterone acetate on uterine leiomyomata volume by magnetic resonance imaging: a prospective, randomized, double blind, placebo-controlled, crossover trial. J Clin Endocrinol Metab 1993;76:1217–23. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

