Rice transcription factor OsMADS25 modulates root growth and confers salinity tolerance via the ABA-mediated regulatory pathway and ROS scavenging
- PMID: 30303953
- PMCID: PMC6197697
- DOI: 10.1371/journal.pgen.1007662
Rice transcription factor OsMADS25 modulates root growth and confers salinity tolerance via the ABA-mediated regulatory pathway and ROS scavenging
Abstract
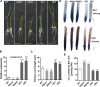
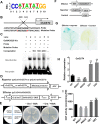
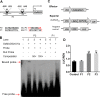
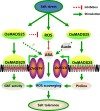
Plant roots are constantly exposed to a variety of abiotic stresses, and high salinity is one of the major limiting conditions that impose constraints on plant growth. In this study, we describe that OsMADS25 is required for the root growth as well as salinity tolerance, via maintaining ROS homeostasis in rice (Oryza sativa). Overexpression of OsMADS25 remarkably enhanced the primary root (PR) length and lateral root (LR) density, whereas RNAi silence of this gene reduced PR elongation significantly, with altered ROS accumulation in the root tip. Transcriptional activation assays indicated that OsMADS25 activates OsGST4 (glutathione S-transferase) expression directly by binding to its promoter. Meanwhile, osgst4 mutant exhibited repressed growth and high sensitivity to salinity and oxidative stress, and recombinant OsGST4 protein was found to have ROS-scavenging activity in vitro. Expectedly, overexpression of OsMADS25 significantly enhanced the tolerance to salinity and oxidative stress in rice plants, with the elevated activity of antioxidant enzymes, increased accumulation of osmoprotective solute proline and reduced frequency of open stoma. Furthermore, OsMADS25 specifically activated the transcription of OsP5CR, a key component of proline biosynthesis, by binding to its promoter. Interestingly, overexpression of OsMADS25 raised the root sensitivity to exogenous ABA, and the expression of ABA-dependent stress-responsive genes was elevated greatly in overexpression plants under salinity stress. In addition, OsMADS25 seemed to promote auxin signaling by activating OsYUC4 transcription. Taken together, our findings reveal that OsMADS25 might be an important transcriptional regulator that regulates the root growth and confers salinity tolerance in rice via the ABA-mediated regulatory pathway and ROS scavenging.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





Similar articles
-
Transcription factor OsMADS25 improves rice tolerance to cold stress.Yi Chuan. 2021 Nov 20;43(11):1078-1087. doi: 10.16288/j.yczz.21-217. Yi Chuan. 2021. PMID: 34815210
-
The cotton WRKY transcription factor GhWRKY17 functions in drought and salt stress in transgenic Nicotiana benthamiana through ABA signaling and the modulation of reactive oxygen species production.Plant Cell Physiol. 2014 Dec;55(12):2060-76. doi: 10.1093/pcp/pcu133. Epub 2014 Sep 26. Plant Cell Physiol. 2014. PMID: 25261532
-
MADS-box transcription factor OsMADS25 regulates root development through affection of nitrate accumulation in rice.PLoS One. 2015 Aug 10;10(8):e0135196. doi: 10.1371/journal.pone.0135196. eCollection 2015. PLoS One. 2015. PMID: 26258667 Free PMC article.
-
Root Development and Stress Tolerance in rice: The Key to Improving Stress Tolerance without Yield Penalties.Int J Mol Sci. 2020 Mar 6;21(5):1807. doi: 10.3390/ijms21051807. Int J Mol Sci. 2020. PMID: 32155710 Free PMC article. Review.
-
Engineering salinity tolerance in plants: progress and prospects.Planta. 2020 Mar 9;251(4):76. doi: 10.1007/s00425-020-03366-6. Planta. 2020. PMID: 32152761 Review.
Cited by
-
Resveratrol Alleviates the KCl Salinity Stress of Malus hupehensis Rhed.Front Plant Sci. 2021 May 12;12:650485. doi: 10.3389/fpls.2021.650485. eCollection 2021. Front Plant Sci. 2021. PMID: 34054896 Free PMC article.
-
Enhancing Crop Resilience: The Role of Plant Genetics, Transcription Factors, and Next-Generation Sequencing in Addressing Salt Stress.Int J Mol Sci. 2024 Nov 22;25(23):12537. doi: 10.3390/ijms252312537. Int J Mol Sci. 2024. PMID: 39684248 Free PMC article. Review.
-
Evolution and Function of MADS-Box Transcription Factors in Plants.Int J Mol Sci. 2024 Dec 11;25(24):13278. doi: 10.3390/ijms252413278. Int J Mol Sci. 2024. PMID: 39769043 Free PMC article. Review.
-
Rice Glycosyltransferase Gene UGT85E1 Is Involved in Drought Stress Tolerance Through Enhancing Abscisic Acid Response.Front Plant Sci. 2021 Dec 23;12:790195. doi: 10.3389/fpls.2021.790195. eCollection 2021. Front Plant Sci. 2021. PMID: 35003178 Free PMC article.
-
Natural variation in ZmNAC087 contributes to total root length regulation in maize seedlings under salt stress.BMC Plant Biol. 2023 Aug 14;23(1):392. doi: 10.1186/s12870-023-04393-7. BMC Plant Biol. 2023. PMID: 37580686 Free PMC article.
References
-
- You J, Zong W, Hu H, Li X, Xiao J, Xiong L (2014) A STRESS-RESPONSIVE NAC1-regulated protein phosphatase pene rice protein phosphatase18 modulates drought and oxidative stress tolerance through abscisic acid-independent reactive oxygen species scavenging in rice. Plant Physiol 166: 2100–2114. 10.1104/pp.114.251116 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

