Transcriptomics reveal potential vaccine antigens and a drastic increase of upregulated genes during Theileria parva development from arthropod to bovine infective stages
- PMID: 30303978
- PMCID: PMC6179218
- DOI: 10.1371/journal.pone.0204047
Transcriptomics reveal potential vaccine antigens and a drastic increase of upregulated genes during Theileria parva development from arthropod to bovine infective stages
Abstract
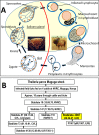
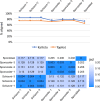
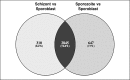
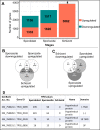
Theileria parva is a protozoan parasite transmitted by the brown ear tick Rhipicephalus appendiculatus that causes East Coast fever (ECF) in cattle, resulting in substantial economic losses in the regions of southern, eastern and central Africa. The schizont form of the parasite transforms the bovine host lymphocytes into actively proliferating cancer-like cells. However, how T. parva causes bovine host cells to proliferate and maintain a cancerous phenotype following infection is still poorly understood. On the other hand, current efforts to develop improved vaccines have identified only a few candidate antigens. In the present paper, we report the first comparative transcriptomic analysis throughout the course of T. parva infection. We observed that the development of sporoblast into sporozoite and then the establishment in the host cells as schizont is accompanied by a drastic increase of upregulated genes in the schizont stage of the parasite. In contrast, the ten highest gene expression values occurred in the arthropod vector stages. A comparative analysis showed that 2845 genes were upregulated in both sporozoite and schizont stages compared to the sporoblast. In addition, 647 were upregulated only in the sporozoite whereas 310 were only upregulated in the schizont. We detected low p67 expression in the schizont stage, an unexpected finding considering that p67 has been reported as a sporozoite stage-specific gene. In contrast, we found that transcription of p67 was 20 times higher in the sporoblast than in the sporozoite. Using the expression profiles of recently identified candidate vaccine antigens as a benchmark for selection for novel potential vaccine candidates, we identified three genes with expression similar to p67 and several other genes similar to Tp1-Tp10 schizont vaccine antigens. We propose that the antigenicity or chemotherapeutic potential of this panel of new candidate antigens be further investigated. Structural comparisons of the transcripts generated here with the existing gene models for the respective loci revealed indels. Our findings can be used to improve the structural annotation of the T. parva genome, and the identification of alternatively spliced transcripts.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



Similar articles
-
Comparative Transcriptomics of the Bovine Apicomplexan Parasite Theileria parva Developmental Stages Reveals Massive Gene Expression Variation and Potential Vaccine Antigens.Front Vet Sci. 2020 Jun 9;7:287. doi: 10.3389/fvets.2020.00287. eCollection 2020. Front Vet Sci. 2020. PMID: 32582776 Free PMC article.
-
Characterization of the Theileria parva sporozoite proteome.Int J Parasitol. 2018 Mar;48(3-4):265-273. doi: 10.1016/j.ijpara.2017.09.007. Epub 2017 Dec 16. Int J Parasitol. 2018. PMID: 29258832 Free PMC article.
-
Genes encoding two Theileria parva antigens recognized by CD8+ T-cells exhibit sequence diversity in South Sudanese cattle populations but the majority of alleles are similar to the Muguga component of the live vaccine cocktail.PLoS One. 2017 Feb 23;12(2):e0171426. doi: 10.1371/journal.pone.0171426. eCollection 2017. PLoS One. 2017. PMID: 28231338 Free PMC article.
-
Immunity and vaccine development in the bovine theilerioses.Adv Parasitol. 1999;44:41-97. doi: 10.1016/s0065-308x(08)60230-4. Adv Parasitol. 1999. PMID: 10563395 Review.
-
Novel vaccines against Theileria parva: prospects for sustainability.Int J Parasitol. 1998 May;28(5):693-706. doi: 10.1016/s0020-7519(98)00011-3. Int J Parasitol. 1998. PMID: 9650049 Review.
Cited by
-
Comparative Transcriptomics of the Bovine Apicomplexan Parasite Theileria parva Developmental Stages Reveals Massive Gene Expression Variation and Potential Vaccine Antigens.Front Vet Sci. 2020 Jun 9;7:287. doi: 10.3389/fvets.2020.00287. eCollection 2020. Front Vet Sci. 2020. PMID: 32582776 Free PMC article.
-
In silico identification of Theileria parva surface proteins.Cell Surf. 2022 May 21;8:100078. doi: 10.1016/j.tcsw.2022.100078. eCollection 2022 Dec. Cell Surf. 2022. PMID: 35647418 Free PMC article.
-
South African Buffalo-Derived Theileria parva Is Distinct From Other Buffalo and Cattle-Derived T. parva.Front Genet. 2021 Jun 25;12:666096. doi: 10.3389/fgene.2021.666096. eCollection 2021. Front Genet. 2021. PMID: 34249088 Free PMC article.
-
The Hypervariable Tpr Multigene Family of Theileria Parasites, Defined by a Conserved, Membrane-Associated, C-Terminal Domain, Includes Several Copies with Defined Orthology Between Species.J Mol Evol. 2023 Dec;91(6):897-911. doi: 10.1007/s00239-023-10142-z. Epub 2023 Nov 28. J Mol Evol. 2023. PMID: 38017120 Free PMC article.
-
Characterization of a Novel Chimeric Theileria parva p67 Antigen Which Incorporates into Virus-like Particles and Is Highly Immunogenic in Mice.Vaccines (Basel). 2022 Jan 28;10(2):210. doi: 10.3390/vaccines10020210. Vaccines (Basel). 2022. PMID: 35214669 Free PMC article.
References
-
- Norval R. A. I., Perry B. D., & Young A. S. (1992). The epidemiology of theileriosis in Africa. ILRI (aka ILCA and ILRAD).
-
- Irvin A.D. and Morrison W.I., 1987. Immunopathology, immunology, and immunoprophylaxis of Theileria infections. Immune responses in parasitic infections: immunology, immunopathology, and immunoprophylaxis. Volume III: Protozoa, pp.223–274.
-
- Morrison W.I., Goddeeris B.M., Brown W.C., Baldwin C.L. and Teale A.J., 1989. Theileria parva in cattle: characterization of infected lymphocytes and the immune responses they provoke. Veterinary immunology and immunopathology, 20(3), pp.213–237. - PubMed
-
- Vollmer D., 2010. Enhancing the effectiveness of sustainability partnerships: Summary of a workshop. National Academies Press.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

