Time resolved 3D live-cell imaging on implants
- PMID: 30304039
- PMCID: PMC6179276
- DOI: 10.1371/journal.pone.0205411
Time resolved 3D live-cell imaging on implants
Abstract
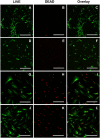
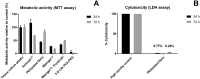
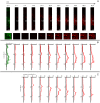
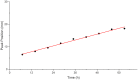
It is estimated that two million new dental implants are inserted worldwide each year. Innovative implant materials are developed in order to minimize the risk of peri-implant inflammations. The broad range of material testing is conducted using standard 2D, terminal, and invasive methods. The methods that have been applied are not sufficient to monitor the whole implant surface and temporal progress. Therefore, we built a 3D peri-implant model using a cylindrical implant colonized by human gingival fibroblasts. In order to monitor the cell response over time, a non-toxic LIVE/DEAD staining was established and applied to the new 3D model. Our LIVE/DEAD staining method in combination with the time resolved 3D visualization using Scanning Laser Optical Tomography (SLOT), allowed us to monitor the cell death path along the implant in the 3D peri-implant model. The differentiation of living and dead gingival fibroblasts in response to toxicity was effectively supported by the LIVE/DEAD staining. Furthermore, it was possible to visualize the whole cell-colonized implant in 3D and up to 63 hours. This new methodology offers the opportunity to record the long-term cell response on external stress factors, along the dental implant and thus to evaluate the performance of novel materials/surfaces.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
Keratinocytes protect soft-tissue integration of dental implant materials against bacterial challenges in a 3D-tissue infection model.Acta Biomater. 2019 Sep 15;96:237-246. doi: 10.1016/j.actbio.2019.07.015. Epub 2019 Jul 11. Acta Biomater. 2019. PMID: 31302293
-
Air atmospheric-pressure plasma-jet treatment enhances the attachment of human gingival fibroblasts for early peri-implant soft tissue seals on titanium dental implant abutments.Acta Odontol Scand. 2015 Jan;73(1):67-75. doi: 10.3109/00016357.2014.954265. Epub 2014 Sep 3. Acta Odontol Scand. 2015. PMID: 25183251
-
Light-microscopic evaluation of the dimensions of peri-implant mucosa around immediately loaded and submerged titanium implants in monkeys.J Periodontol. 2008 Sep;79(9):1697-703. doi: 10.1902/jop.2008.070631. J Periodontol. 2008. PMID: 18771371
-
Novel digital imaging techniques to assess the outcome in oral rehabilitation with dental implants: a narrative review.Clin Oral Implants Res. 2015 Sep;26 Suppl 11:86-96. doi: 10.1111/clr.12616. Epub 2015 May 26. Clin Oral Implants Res. 2015. PMID: 26010421 Review.
-
Comparison of peri-implant and periodontal marginal soft tissues in health and disease.Periodontol 2000. 2018 Feb;76(1):116-130. doi: 10.1111/prd.12150. Epub 2017 Nov 30. Periodontol 2000. 2018. PMID: 29193334 Review.
Cited by
-
Stimulation of Osteogenic Activity of Autologous Teeth Hard Tissues as Bone Augmentation Material.Biology (Basel). 2024 Jan 11;13(1):40. doi: 10.3390/biology13010040. Biology (Basel). 2024. PMID: 38248471 Free PMC article.
References
-
- Dreyer H, Grischke J, Tiede C, Eberhard J, Schweitzer A, Toikkanen SE, et al. Epidemiology and risk factors of peri-implantitis: A systematic review. J Periodontal Res. 2018: Forthcoming. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

