Peanut-specific T cell responses in patients with different clinical reactivity
- PMID: 30304054
- PMCID: PMC6179248
- DOI: 10.1371/journal.pone.0204620
Peanut-specific T cell responses in patients with different clinical reactivity
Abstract
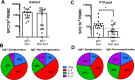
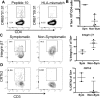
Whole extract or allergen-specific IgE testing has become increasingly popular in the diagnosis of peanut allergy. However, much less is known about T cell responses in peanut allergy and how it relates to different clinical phenotypes. CD4+ T cells play a major role in the pathophysiology of peanut allergy as well as tolerance induction during oral desensitization regimens. We set out to characterize and phenotype the T cell responses and their targets in peanut sensitized patients. Using PBMC from peanut-allergic and non-allergic patients, we mapped T cell epitopes for three major peanut allergens, Ara h 1, 2 and 3 (27 from Ara h 1, 4 from Ara h 2 and 43 from Ara h 3) associated with release of IFNγ (representative Th1 cytokine) and IL5 (representative Th2 cytokine). A pool containing 19 immunodominant peptides, selected to account for 60% of the total Ara h 1-3-specific T cell response in allergics, but only 20% in non-allergics, was shown to discriminate T cell responses in peanut-sensitized, symptomatic vs non-symptomatic individuals more effectively than peanut extract. This pool elicited positive T cell responses above a defined threshold in 12/15 sensitized, symptomatic patients, whereas in the sensitized but non-symptomatic cohort only, 4/14 reacted. The reactivity against this peptide pool in symptomatic patients was dominated by IL-10, IL-17 and to a lesser extend IL-5. For four distinct epitopes, HLA class II restrictions were determined, enabling production of tetrameric reagents. Tetramer staining in four donors (2 symptomatic, 2 non-symptomatic) revealed a trend for increased numbers of peanut epitope-specific T cells in symptomatic patients compared to non-symptomatic patients, which was associated with elevated CRTh2 expression whereas cells from non-symptomatic patients exhibited higher levels of Integrin β7 expression. Our results demonstrate differences in T cell response magnitude, epitope specificity and phenotype between symptomatic and non-symptomatic peanut-sensitized patients. In addition to IgE reactivity, analysis of peanut-specific T cells may be useful to improve our understanding of different clinical manifestations in peanut allergy.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Ara h 1-reactive T cells in individuals with peanut allergy.J Allergy Clin Immunol. 2011 May;127(5):1211-8.e3. doi: 10.1016/j.jaci.2011.02.028. Epub 2011 Apr 2. J Allergy Clin Immunol. 2011. PMID: 21459424 Free PMC article.
-
Ara h 1 CD4+ T cell epitope-based peptides: candidates for a peanut allergy therapeutic.Clin Exp Allergy. 2013 Jun;43(6):684-97. doi: 10.1111/cea.12113. Clin Exp Allergy. 2013. PMID: 23711131 Free PMC article.
-
Analysis of cytokine production by peanut-reactive T cells identifies residual Th2 effectors in highly allergic children who received peanut oral immunotherapy.Clin Exp Allergy. 2015 Jul;45(7):1201-13. doi: 10.1111/cea.12537. Clin Exp Allergy. 2015. PMID: 25823600 Free PMC article.
-
Redefining the major peanut allergens.Immunol Res. 2013 Mar;55(1-3):125-34. doi: 10.1007/s12026-012-8355-x. Immunol Res. 2013. PMID: 22948807 Free PMC article. Review.
-
Proteomic screening points to the potential importance of Ara h 3 basic subunit in allergenicity of peanut.Inflamm Allergy Drug Targets. 2008 Sep;7(3):163-6. doi: 10.2174/187152808785748182. Inflamm Allergy Drug Targets. 2008. PMID: 18782022 Review.
Cited by
-
Oral desensitization therapy for peanut allergy induces dynamic changes in peanut-specific immune responses.Allergy. 2022 Aug;77(8):2534-2548. doi: 10.1111/all.15276. Epub 2022 Mar 14. Allergy. 2022. PMID: 35266148 Free PMC article. Clinical Trial.
-
Development and Validation of a Bordetella pertussis Whole-Genome Screening Strategy.J Immunol Res. 2020 Apr 2;2020:8202067. doi: 10.1155/2020/8202067. eCollection 2020. J Immunol Res. 2020. PMID: 32322598 Free PMC article.
-
Molecular Allergology: Epitope Discovery and Its Application for Allergen-Specific Immunotherapy of Food Allergy.Clin Rev Allergy Immunol. 2025 Apr 8;68(1):37. doi: 10.1007/s12016-025-09052-3. Clin Rev Allergy Immunol. 2025. PMID: 40198416 Review.
-
T-cell epitope discovery and single-cell technologies to advance food allergy research.J Allergy Clin Immunol. 2023 Jan;151(1):15-20. doi: 10.1016/j.jaci.2022.10.025. Epub 2022 Nov 18. J Allergy Clin Immunol. 2023. PMID: 36411114 Free PMC article. Review.
-
In Silico Identification of Peanut Peptides Suitable for Allergy Immunotherapy in HLA-DRB1*03:01-Restricted Patients.Pharmaceuticals (Basel). 2024 Aug 21;17(8):1097. doi: 10.3390/ph17081097. Pharmaceuticals (Basel). 2024. PMID: 39204201 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

