Ultrastructural viewpoints on the interaction events of Scedosporium apiospermum conidia with lung and macrophage cells
- PMID: 30304087
- PMCID: PMC6174640
- DOI: 10.1590/0074-02760180311
Ultrastructural viewpoints on the interaction events of Scedosporium apiospermum conidia with lung and macrophage cells
Abstract
Background: Scedosporium apiospermum is a ubiquitous, emerging and multidrug-resistant fungal pathogen with still rather unknown virulence mechanisms.
Objectives/methods: The cellular basis of the in vitro interaction between fungi and host cells/tissues is the determinant factor for the development of a successful in vivo infection. Herein, we evaluated the interaction of S. apiospermum conidia with lung epithelial (A549), lung fibroblast (MRC-5) and RAW 264.7 macrophages by light and scanning/transmission electron microscopy.
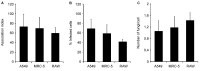
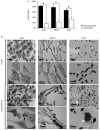
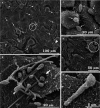
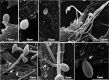
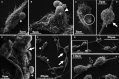
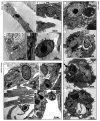
Findings: After 4 h of fungi-host cell contact, the percentage of infected mammalian cells and the number of fungi per infected cell was measured by light microscopy, and the following association indexes were calculated for A549, MRC-5 and macrophage cells: 73.2 ± 25.9, 69.7 ± 22.5 and 59.7 ± 11.1, respectively. Both conidia and germinated conidia were regularly observed interacting with the evaluated cells, with a higher prevalence of non-germinated conidia. Interestingly, nests of germinated conidia were evidenced at the surface of lung cells by scanning electron microscopy. Some germination projections and hyphae were seen penetrating/evading the mammalian cells. Furthermore, internalised conidia were seen within vacuoles as visualised by transmission electron microscopy.
Main conclusions: The present study contributes to a better understanding of S. apiospermum pathogenesis by demonstrating the first steps of the infection process of this opportunistic fungus.
Figures
Similar articles
-
Insights into the interaction of Scedosporium apiospermum, Scedosporium aurantiacum, Scedosporium minutisporum, and Lomentospora prolificans with lung epithelial cells.Braz J Microbiol. 2020 Jun;51(2):427-436. doi: 10.1007/s42770-019-00183-2. Epub 2019 Nov 17. Braz J Microbiol. 2020. PMID: 31736016 Free PMC article.
-
Assessment of biofilm formation by Scedosporium apiospermum, S. aurantiacum, S. minutisporum and Lomentospora prolificans.Biofouling. 2016 Aug;32(7):737-49. doi: 10.1080/08927014.2016.1192610. Biofouling. 2016. PMID: 27309801
-
Scedosporium apiospermum, Scedosporium aurantiacum, Scedosporium minutisporum and Lomentospora prolificans: a comparative study of surface molecules produced by conidial and germinated conidial cells.Mem Inst Oswaldo Cruz. 2018;113(6):e180102. doi: 10.1590/0074-02760180102. Epub 2018 Jun 18. Mem Inst Oswaldo Cruz. 2018. PMID: 29924142 Free PMC article.
-
Scedosporium apiospermum infectious scleritis following posterior subtenon triamcinolone acetonide injection: a case report and literature review.BMC Ophthalmol. 2018 Feb 13;18(1):40. doi: 10.1186/s12886-018-0707-4. BMC Ophthalmol. 2018. PMID: 29433463 Free PMC article. Review.
-
Enzymatic Mechanisms Involved in Evasion of Fungi to the Oxidative Stress: Focus on Scedosporium apiospermum.Mycopathologia. 2018 Feb;183(1):227-239. doi: 10.1007/s11046-017-0160-6. Epub 2017 Jun 21. Mycopathologia. 2018. PMID: 28639066 Review.
Cited by
-
Interactions of an Emerging Fungal Pathogen Scedosporium aurantiacum with Human Lung Epithelial Cells.Sci Rep. 2019 Mar 22;9(1):5035. doi: 10.1038/s41598-019-41435-3. Sci Rep. 2019. PMID: 30903006 Free PMC article.
-
Impact of Copper(II) and Silver(I) Complexes Containing 1,10-phenanthroline-5,6-dione on Cellular and Virulence Aspects of Scedosporium apiospermum.Curr Top Med Chem. 2025;25(11):1377-1388. doi: 10.2174/0115680266327984241018111547. Curr Top Med Chem. 2025. PMID: 39754777
-
Cytoskeletal Alteration Is an Early Cellular Response in Pulmonary Epithelium Infected with Aspergillus fumigatus Rather than Scedosporium apiospermum.Microb Ecol. 2022 Jan;83(1):216-235. doi: 10.1007/s00248-021-01750-7. Epub 2021 Apr 22. Microb Ecol. 2022. PMID: 33890146
-
Fibronectin-binding molecules of Scedosporium apiospermum: focus on adhesive events.Braz J Microbiol. 2023 Dec;54(4):2577-2585. doi: 10.1007/s42770-023-01062-7. Epub 2023 Jul 13. Braz J Microbiol. 2023. PMID: 37442880 Free PMC article.
-
Insights into the interaction of Scedosporium apiospermum, Scedosporium aurantiacum, Scedosporium minutisporum, and Lomentospora prolificans with lung epithelial cells.Braz J Microbiol. 2020 Jun;51(2):427-436. doi: 10.1007/s42770-019-00183-2. Epub 2019 Nov 17. Braz J Microbiol. 2020. PMID: 31736016 Free PMC article.
References
-
- Mello TP, Bittencourt VCB, Liporagi-Lopes LC, Aor AC, Branquinha MH, Santos ALS. Insights into the social life and obscure side of Scedosporium/Lomentospora species: ubiquitous, emerging and multidrug-resistant opportunistic pathogens. Fungal Biol Rev. 2018
-
- Hornbeek H, Ackerman BH, Reigart CL, Stair-Buchmann M, Guilday RE, Patton ML. Pseudallescheria boydii infection of the brain. Surg Infect. 2012;13(3):179–180. - PubMed
-
- Slavin M, van Hal S, Sorrell TC, Lee A, Marriott DJ, Daveson K, et al. Australia and New Zealand Mycoses Interest Group. Invasive infections due to filamentous fungi other than Aspergillus: epidemiology and determinants of mortality. Clin Microbiol Infect. 2015;21(5):490.e1–490.10. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

