Oral dydrogesterone versus intravaginal micronized progesterone gel for luteal phase support in IVF: a randomized clinical trial
- PMID: 30304457
- PMCID: PMC6238366
- DOI: 10.1093/humrep/dey306
Oral dydrogesterone versus intravaginal micronized progesterone gel for luteal phase support in IVF: a randomized clinical trial
Abstract
Study question: Is oral dydrogesterone 30 mg daily non-inferior to 8% micronized vaginal progesterone (MVP) gel 90 mg daily for luteal phase support in IVF?
Summary answer: Oral dydrogesterone demonstrated non-inferiority to MVP gel for the presence of fetal heartbeats at 12 weeks of gestation (non-inferiority margin 10%).
What is known already: The standard of care for luteal phase support in IVF is the use of MVP; however, it is associated with vaginal irritation, discharge and poor patient compliance. Oral dydrogesterone may replace MVP as the standard of care if it is found to be efficacious with an acceptable safety profile.
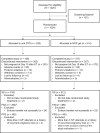
Study design, size, duration: Lotus II was a randomized, open-label, multicenter, Phase III, non-inferiority study conducted at 37 IVF centers in 10 countries worldwide, from August 2015 until May 2017. In total, 1034 premenopausal women (>18 to <42 years of age) undergoing IVF were randomized 1:1 (stratified by country and age group), using an Interactive Web Response System, to receive oral dydrogesterone 30 mg or 8% MVP gel 90 mg daily.
Participants/materials, setting, methods: Subjects received either oral dydrogesterone (n = 520) or MVP gel (n = 514) on the day of oocyte retrieval, and luteal phase support continued until 12 weeks of gestation. The primary outcome measure was the presence of fetal heartbeats at 12 weeks of gestation, as determined by transvaginal ultrasound.
Main results and the role of chance: Non-inferiority of oral dydrogesterone was demonstrated, with pregnancy rates in the full analysis sample (FAS) at 12 weeks of gestation of 38.7% (191/494) and 35.0% (171/489) in the oral dydrogesterone and MVP gel groups, respectively (adjusted difference, 3.7%; 95% CI: -2.3 to 9.7). Live birth rates in the FAS of 34.4% (170/494) and 32.5% (159/489) were obtained for the oral dydrogesterone and MVP gel groups, respectively (adjusted difference 1.9%; 95% CI: -4.0 to 7.8). Oral dydrogesterone was well tolerated and had a similar safety profile to MVP gel.
Limitations, reasons for caution: The analysis of the results was powered to consider the ongoing pregnancy rate, but a primary objective of greater clinical interest may have been the live birth rate. This study was open-label as it was not technically feasible to make a placebo applicator for MVP gel, which may have increased the risk of bias for the subjective endpoints reported in this study. While the use of oral dydrogesterone in fresh-cycle IVF was investigated in this study, further research is needed to investigate its efficacy in programmed frozen-thawed cycles where corpora lutea do not exist.
Wider implications of the findings: This study demonstrates that oral dydrogesterone is a viable alternative to MVP gel, due to its comparable efficacy and tolerability profiles. Owing to its patient-friendly oral administration route, dydrogesterone may replace MVP as the standard of care for luteal phase support in fresh-cycle IVF.
Study funding/competing interests(s): This study was sponsored and supported by Abbott. G.G. has received investigator fees from Abbott during the conduct of the study. Outside of this submitted work, G.G. has received non-financial support from MSD, Ferring, Merck-Serono, IBSA, Finox, TEVA, Glycotope and Gedeon Richter, as well as personal fees from MSD, Ferring, Merck-Serono, IBSA, Finox, TEVA, Glycotope, VitroLife, NMC Healthcare, ReprodWissen, Biosilu, Gedeon Richter and ZIVA. C.B. is the President of the Belgian Society of Reproductive Medicine (unpaid) and Section Editor of Reproductive BioMedicine Online. C.B. has received grants from Ferring Pharmaceuticals, participated in an MSD sponsored trial, and has received payment from Ferring, MSD, Biomérieux, Abbott and Merck for lectures. G.S. has no conflicts of interest to be declared. A.P. is the General Secretary of the Indian Society of Assisted Reproduction (2017-2018). B.D. is President of Pune Obstetric and Gynecological Society (2017-2018). D.-Z.Y. has no conflicts of interest to be declared. Z.-J.C. has no conflicts of interest to be declared. E.K. is an employee of Abbott Laboratories GmbH, Hannover, Germany and owns shares in Abbott. C.P.-F. is an employee of Abbott GmbH & Co. KG, Wiesbaden, Germany and owns shares in Abbott. H.T.'s institution has received grants from Merck, MSD, Goodlife, Cook, Roche, Origio, Besins, Ferring and Mithra (now Allergan); and H.T. has received consultancy fees from Finox-Gedeon Richter, Merck, Ferring, Abbott and ObsEva.
Trial registration number: NCT02491437 (clinicaltrials.gov).
Trial registration date: 08 July 2015.
Date of first patient’s enrollment: 17 August 2015.
Figures

References
-
- Arvidsson C, Hellborg M, Gemzell-Danielsson K. Preference and acceptability of oral versus vaginal administration of misoprostol in medical abortion with mifepristone. Eur J Obstet Gynecol Reprod Biol 2005;123:87–91. - PubMed
-
- Barbosa MW, Silva LR, Navarro PA, Ferriani RA, Nastri CO, Martins WP. Dydrogesterone vs progesterone for luteal-phase support: systematic review and meta-analysis of randomized controlled trials. Ultrasound Obstet Gynecol 2016;48:161–170. - PubMed
-
- Besins Healthcare (UK) Ltd Utrogestan 100 mg capsules Summary of Product Characteristics, 14 July 2017.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

