Imaging Advances in Chronic Obstructive Pulmonary Disease. Insights from the Genetic Epidemiology of Chronic Obstructive Pulmonary Disease (COPDGene) Study
- PMID: 30304637
- PMCID: PMC6363977
- DOI: 10.1164/rccm.201807-1351SO
Imaging Advances in Chronic Obstructive Pulmonary Disease. Insights from the Genetic Epidemiology of Chronic Obstructive Pulmonary Disease (COPDGene) Study
Abstract
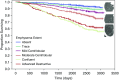
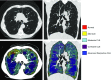
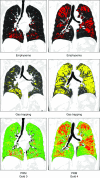
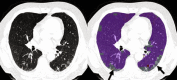
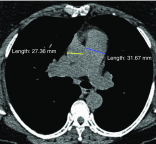
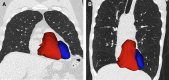
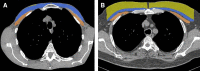
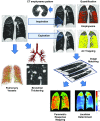
The Genetic Epidemiology of Chronic Obstructive Pulmonary Disease (COPDGene) study, which began in 2007, is an ongoing multicenter observational cohort study of more than 10,000 current and former smokers. The study is aimed at understanding the etiology, progression, and heterogeneity of chronic obstructive pulmonary disease (COPD). In addition to genetic analysis, the participants have been extensively characterized by clinical questionnaires, spirometry, volumetric inspiratory and expiratory computed tomography, and longitudinal follow-up, including follow-up computed tomography at 5 years after enrollment. The purpose of this state-of-the-art review is to summarize the major advances in our understanding of COPD resulting from the imaging findings in the COPDGene study. Imaging features that are associated with adverse clinical outcomes include early interstitial lung abnormalities, visual presence and pattern of emphysema, the ratio of pulmonary artery to ascending aortic diameter, quantitative evaluation of emphysema, airway wall thickness, and expiratory gas trapping. COPD is characterized by the early involvement of the small conducting airways, and the addition of expiratory scans has enabled measurement of small airway disease. Computational advances have enabled indirect measurement of nonemphysematous gas trapping. These metrics have provided insights into the pathogenesis and prognosis of COPD and have aided early identification of disease. Important quantifiable extrapulmonary findings include coronary artery calcification, cardiac morphology, intrathoracic and extrathoracic fat, and osteoporosis. Current active research includes identification of novel quantitative measures for emphysema and airway disease, evaluation of dose reduction techniques, and use of deep learning for phenotyping COPD.
Keywords: chronic obstructive pulmonary disease; computed tomography; lung imaging.
Figures
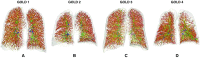
References
-
- Kirby M, van Beek EJR, Seo JB, Biederer J, Nakano Y, Coxson HO, et al. Management of COPD: is there a role for quantitative imaging? Eur J Radiol. 2017;86:335–342. - PubMed

