Classification, Ontology, and Precision Medicine
- PMID: 30304648
- PMCID: PMC6503847
- DOI: 10.1056/NEJMra1615014
Classification, Ontology, and Precision Medicine
Figures
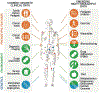
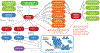


Comment in
-
Undiagnosed and rare diseases: current challenges, perspectives and contribution of oral cavity examination.Oral Surg Oral Med Oral Pathol Oral Radiol. 2020 Aug;130(2):227-228. doi: 10.1016/j.oooo.2020.04.011. Epub 2020 May 31. Oral Surg Oral Med Oral Pathol Oral Radiol. 2020. PMID: 32493682 No abstract available.
References
-
- National Research Council, Committee on a Framework for Developing a New Taxonomy of Disease. Toward precision medicine: building a knowledge network for biomedical research and a new taxonomy of disease. Washington, DC: National Academies Press, 2011. - PubMed
-
- Marx V The DNA of a nation. Nature 2015;524:503–5. - PubMed
-
- Goroll AH. Emerging from EHR purgatory — moving from process to outcomes. N Engl J Med 2017;376:2004–6. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
