Classification and Personalized Prognosis in Myeloproliferative Neoplasms
- PMID: 30304655
- PMCID: PMC7030948
- DOI: 10.1056/NEJMoa1716614
Classification and Personalized Prognosis in Myeloproliferative Neoplasms
Abstract
Background: Myeloproliferative neoplasms, such as polycythemia vera, essential thrombocythemia, and myelofibrosis, are chronic hematologic cancers with varied progression rates. The genomic characterization of patients with myeloproliferative neoplasms offers the potential for personalized diagnosis, risk stratification, and treatment.
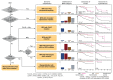
Methods: We sequenced coding exons from 69 myeloid cancer genes in patients with myeloproliferative neoplasms, comprehensively annotating driver mutations and copy-number changes. We developed a genomic classification for myeloproliferative neoplasms and multistage prognostic models for predicting outcomes in individual patients. Classification and prognostic models were validated in an external cohort.
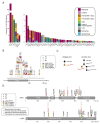
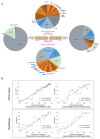
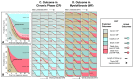
Results: A total of 2035 patients were included in the analysis. A total of 33 genes had driver mutations in at least 5 patients, with mutations in JAK2, CALR, or MPL being the sole abnormality in 45% of the patients. The numbers of driver mutations increased with age and advanced disease. Driver mutations, germline polymorphisms, and demographic variables independently predicted whether patients received a diagnosis of essential thrombocythemia as compared with polycythemia vera or a diagnosis of chronic-phase disease as compared with myelofibrosis. We defined eight genomic subgroups that showed distinct clinical phenotypes, including blood counts, risk of leukemic transformation, and event-free survival. Integrating 63 clinical and genomic variables, we created prognostic models capable of generating personally tailored predictions of clinical outcomes in patients with chronic-phase myeloproliferative neoplasms and myelofibrosis. The predicted and observed outcomes correlated well in internal cross-validation of a training cohort and in an independent external cohort. Even within individual categories of existing prognostic schemas, our models substantially improved predictive accuracy.
Conclusions: Comprehensive genomic characterization identified distinct genetic subgroups and provided a classification of myeloproliferative neoplasms on the basis of causal biologic mechanisms. Integration of genomic data with clinical variables enabled the personalized predictions of patients' outcomes and may support the treatment of patients with myeloproliferative neoplasms. (Funded by the Wellcome Trust and others.).
Figures

Comment in
-
New Classification for Myeloproliferative Neoplasms.Cancer Discov. 2018 Dec;8(12):OF1. doi: 10.1158/2159-8290.CD-NB2018-144. Epub 2018 Nov 8. Cancer Discov. 2018. PMID: 30409777
References
-
- Dameshek W. Some speculations on the myeloproliferative syndromes. Blood. 1951;6(4):372–5. - PubMed
-
- Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391–405. - PubMed
-
- Harrison CN, Butt N, Campbell P, et al. Modification of British Committee for Standards in Haematology diagnostic criteria for essential thrombocythaemia. Br J Haematol. 2014;167(3):421–3. - PubMed
-
- McMullin MF, Reilly JT, Campbell P, et al. Amendment to the guideline for diagnosis and investigation of polycythaemia/erythrocytosis. Br J Haematol. 2007;138(6):821–2. - PubMed
-
- Reilly JT, McMullin MF, Beer PA, et al. Use of JAK inhibitors in the management of myelofibrosis: a revision of the British Committee for Standards in Haematology Guidelines for Investigation and Management of Myelofibrosis 2012. Br J Haematol. 2014;167(3):418–20. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
