The 10,000 Immunomes Project: Building a Resource for Human Immunology
- PMID: 30304689
- PMCID: PMC6263160
- DOI: 10.1016/j.celrep.2018.09.021
The 10,000 Immunomes Project: Building a Resource for Human Immunology
Erratum in
-
The 10,000 Immunomes Project: Building a Resource for Human Immunology.Cell Rep. 2018 Nov 13;25(7):1995. doi: 10.1016/j.celrep.2018.11.013. Cell Rep. 2018. PMID: 30428364 Free PMC article. No abstract available.
Abstract
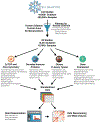
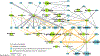
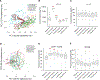
There is increasing appreciation that the immune system plays critical roles not only in the traditional domains of infection and inflammation but also in many areas of biology, including tumorigenesis, metabolism, and even neurobiology. However, one of the major barriers for understanding human immunological mechanisms is that immune assays have not been reproducibly characterized for a sufficiently large and diverse healthy human cohort. Here, we present the 10,000 Immunomes Project (10KIP), a framework for growing a diverse human immunology reference, from ImmPort, a publicly available resource of subject-level immunology data. Although some measurement types are sparse in the presently deposited ImmPort database, the extant data allow for a diversity of robust comparisons. Using 10KIP, we describe variations in serum cytokines and leukocytes by age, race, and sex; define a baseline cell-cytokine network; and describe immunologic changes in pregnancy. All data in the resource are available for visualization and download at http://10kimmunomes.org/.
Copyright © 2018 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
DECLARATION OF INTERESTS
The authors declare no competing interests.
Figures


References
-
- Ashley EA (2015). The precision medicine initiative: a new national effort. JAMA 313, 2119–2120. - PubMed
-
- Becher B, Spath S, and Goverman J (2017). Cytokine networks in neuroinflammation. Nat. Rev. Immunol 17, 49–59. - PubMed
-
- Belkaid Y, and Rouse BT (2005). Natural regulatoryTcells in infectious disease. Nat. Immunol 6, 353–360. - PubMed

