Effect of Low-Fat Diet in Obese Mice Lacking Toll-like Receptors
- PMID: 30304787
- PMCID: PMC6213519
- DOI: 10.3390/nu10101464
Effect of Low-Fat Diet in Obese Mice Lacking Toll-like Receptors
Abstract
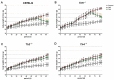
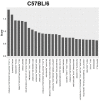
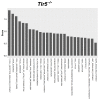
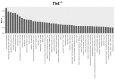
Background: This study aimed at assessing the effect of a low-fat diet (LFD) in obese mice lacking toll⁻like receptors (Tlr) and understanding the expression and regulation of microRNAs during weight reduction. Methods: C57BL/6, Tlr5-/-, Tlr2-/- and Tlr4-/- mice were used in this study. A group of mice were fed with a high-fat diet (HFD) (58% kcal) for 12 weeks to induce obesity (diet-induced obesity, DIO). Another group that had been fed with HFD for eight weeks (obese mice) were switched to a low-fat diet (LFD) (10.5% kcal) for the next four weeks to reduce their body weight. The control mice were fed with a standard AIN-76A diet for the entire 12 weeks. The body weight of the mice was measured weekly. At the end of the experiment, epididymal fat weight and adipocyte size were measured. The differentially expressed miRNAs in the fat tissue was determined by next-generation sequencing with real-time quantitative reverse transcription polymerase chain reaction (RT⁻qPCR). Target prediction and functional annotation of miRNAs were performed using miRSystem database. Results: Switching to LFD significantly reduced the body weight and epididymal fat mass in the HFD-fed C57BL/6 and Tlr5-/- mice but not in Tlr2-/- and Tlr4-/- mice. Weight reduction significantly decreased the size of adipocytes in C57BL/6 but not in the Tlr knockout mice. In Tlr2-/- and Tlr4-/- mice, feeding with HFD and the subsequent weight reduction resulted in an aberrant miRNA expression in the epididymal fat tissue unlike in C57BL/6 and Tlr5-/-. However, target prediction and functional annotation by miRSystem database revealed that all the top 10 Kyoto Encyclopedia of Genes and Genomes (KEGG) database pathways of the dysregulated miRNAs during weight reduction in the C57BL/6 mice were also found in the regulated pathways of Tlr5-/-, Tlr2-/-, and Tlr4-/- strains. However, among these pathways, gene sets involved in arginine and proline metabolism and glutathione metabolism were mainly involved in the Tlr knockout mice but not in the C57BL/6 mice. Conclusions: In this study, we demonstrated that feeding of LFD leads to significant body weight reduction in C57BL/6 and Tlr5-/- mice, but not in Tlr2-/- and Tlr4-/- mice. Significant reduction in the size of adipocytes of epididymal fat was only found in C57BL/6, but not in Tlr5-/-, Tlr2-/-, and Tlr4-/- mice. The dysregulated miRNAs in Tlr2-/- and Tlr4-/- mice were different from those in C57BL/6 and Tlr5-/- strains. Among those miRNA-regulated pathways, arginine and proline metabolism as well as glutathione metabolism may have important roles in the Tlr knockout mice rather than in C57BL/6 mice.
Keywords: diet-induced obesity; high-fat diet; low-fat diet; toll-like receptor; weight reduction.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Similar articles
-
Effect of Weight-Reduction in Obese Mice Lacking Toll-Like Receptor 5 and C57BL/6 Mice Fed a Low-Fat Diet.Mediators Inflamm. 2015;2015:852126. doi: 10.1155/2015/852126. Epub 2015 Nov 23. Mediators Inflamm. 2015. PMID: 26681840 Free PMC article.
-
Weight-reduction through a low-fat diet causes differential expression of circulating microRNAs in obese C57BL/6 mice.BMC Genomics. 2015 Sep 16;16(1):699. doi: 10.1186/s12864-015-1896-3. BMC Genomics. 2015. PMID: 26377847 Free PMC article.
-
Carvacrol prevents diet-induced obesity by modulating gene expressions involved in adipogenesis and inflammation in mice fed with high-fat diet.J Nutr Biochem. 2012 Feb;23(2):192-201. doi: 10.1016/j.jnutbio.2010.11.016. Epub 2011 Mar 29. J Nutr Biochem. 2012. PMID: 21447440
-
Epigenetic regulation of Toll-like receptors 2 and 4 in kidney disease.J Mol Med (Berl). 2022 Jul;100(7):1017-1026. doi: 10.1007/s00109-022-02218-y. Epub 2022 Jun 15. J Mol Med (Berl). 2022. PMID: 35704060 Review.
-
Role of toll-like receptor in the pathogenesis of oral cancer.Cell Biochem Biophys. 2024 Mar;82(1):91-105. doi: 10.1007/s12013-023-01191-8. Epub 2023 Oct 19. Cell Biochem Biophys. 2024. PMID: 37853249 Review.
Cited by
-
Dietary Protection against Cognitive Impairment, Neuroinflammation and Oxidative Stress in Alzheimer's Disease Animal Models of Lipopolysaccharide-Induced Inflammation.Int J Mol Sci. 2023 Mar 21;24(6):5921. doi: 10.3390/ijms24065921. Int J Mol Sci. 2023. PMID: 36982996 Free PMC article. Review.
-
Innate-Immunity Genes in Obesity.J Pers Med. 2021 Nov 14;11(11):1201. doi: 10.3390/jpm11111201. J Pers Med. 2021. PMID: 34834553 Free PMC article. Review.
-
Hesperetin, a Citrus Flavonoid, Attenuates LPS-Induced Neuroinflammation, Apoptosis and Memory Impairments by Modulating TLR4/NF-κB Signaling.Nutrients. 2019 Mar 17;11(3):648. doi: 10.3390/nu11030648. Nutrients. 2019. PMID: 30884890 Free PMC article.
-
Meisoindigo Protects Against Focal Cerebral Ischemia-Reperfusion Injury by Inhibiting NLRP3 Inflammasome Activation and Regulating Microglia/Macrophage Polarization via TLR4/NF-κB Signaling Pathway.Front Cell Neurosci. 2019 Dec 16;13:553. doi: 10.3389/fncel.2019.00553. eCollection 2019. Front Cell Neurosci. 2019. PMID: 31920554 Free PMC article.
-
TIR-Domain-Containing Adaptor-Inducing Interferon-β (TRIF) Is Involved in Glucose Metabolism in Adipose Tissue through the Insulin/AKT Signaling Pathway.Int J Endocrinol. 2020 Dec 9;2020:6942307. doi: 10.1155/2020/6942307. eCollection 2020. Int J Endocrinol. 2020. PMID: 33376487 Free PMC article.
References
-
- Engin A.B. Adipocyte-Macrophage Cross-Talk in Obesity. Adv. Exp. Med. Biol. 2017;960:327–343. - PubMed
-
- Omagari K., Kato S., Tsuneyama K., Inohara C., Kuroda Y., Tsukuda H., Fukazawa E., Shiraishi K., Mune M. Effects of a long-term high-fat diet and switching from a high-fat to low-fat, standard diet on hepatic fat accumulation in Sprague-Dawley rats. Dig. Dis. Sci. 2008;53:3206–3212. doi: 10.1007/s10620-008-0303-1. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

