Strain-Dependent Consequences of Zika Virus Infection and Differential Impact on Neural Development
- PMID: 30304805
- PMCID: PMC6212967
- DOI: 10.3390/v10100550
Strain-Dependent Consequences of Zika Virus Infection and Differential Impact on Neural Development
Abstract
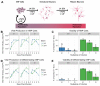
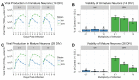
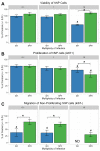
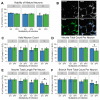
Maternal infection with Zika virus (ZIKV) during pregnancy can result in neonatal abnormalities, including neurological dysfunction and microcephaly. Experimental models of congenital Zika syndrome identified neural progenitor cells as a target of viral infection. Neural progenitor cells are responsible for populating the developing central nervous system with neurons and glia. Neural progenitor dysfunction can lead to severe birth defects, namely, lissencephaly, microcephaly, and cognitive deficits. For this study, the consequences of ZIKV infection in human pluripotent stem cell-derived neural progenitor (hNP) cells and neurons were evaluated. ZIKV isolates from Asian and African lineages displayed lineage-specific replication kinetics, cytopathic effects, and impacts on hNP function and neuronal differentiation. The currently circulating ZIKV isolates exhibit a unique profile of virulence, cytopathic effect, and impaired cellular functions that likely contribute to the pathological mechanism of congenital Zika syndrome. The authors found that infection with Asian-lineage ZIKV isolates impaired the proliferation and migration of hNP cells, and neuron maturation. In contrast, the African-lineage infections resulted in abrupt and extensive cell death. This work furthers the understanding of ZIKV-induced brain pathology.
Keywords: Zika virus; neural progenitor cells; neurons.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; and in the decision to publish the results.
Figures

Similar articles
-
Infectivity of Immature Neurons to Zika Virus: A Link to Congenital Zika Syndrome.EBioMedicine. 2016 Aug;10:65-70. doi: 10.1016/j.ebiom.2016.06.026. Epub 2016 Jun 23. EBioMedicine. 2016. PMID: 27364784 Free PMC article.
-
Zika Virus Induces Tumor Necrosis Factor-Related Apoptosis Inducing Ligand (TRAIL)-Mediated Apoptosis in Human Neural Progenitor Cells.Cells. 2020 Nov 16;9(11):2487. doi: 10.3390/cells9112487. Cells. 2020. PMID: 33207682 Free PMC article.
-
Zika infection and the development of neurological defects.Cell Microbiol. 2017 Jun;19(6). doi: 10.1111/cmi.12744. Epub 2017 May 3. Cell Microbiol. 2017. PMID: 28370966 Review.
-
Zika Virus Infection Induces DNA Damage Response in Human Neural Progenitors That Enhances Viral Replication.J Virol. 2019 Sep 30;93(20):e00638-19. doi: 10.1128/JVI.00638-19. Print 2019 Oct 15. J Virol. 2019. PMID: 31375586 Free PMC article.
-
The impact of Zika virus in the brain.Biochem Biophys Res Commun. 2017 Oct 28;492(4):603-607. doi: 10.1016/j.bbrc.2017.01.074. Epub 2017 Jan 17. Biochem Biophys Res Commun. 2017. PMID: 28108286 Review.
Cited by
-
Using Macaques to Address Critical Questions in Zika Virus Research.Annu Rev Virol. 2019 Sep 29;6(1):481-500. doi: 10.1146/annurev-virology-092818-015732. Epub 2019 Jun 10. Annu Rev Virol. 2019. PMID: 31180813 Free PMC article. Review.
-
Patterns of Herpes Simplex Virus 1 Infection in Neural Progenitor Cells.J Virol. 2020 Jul 30;94(16):e00994-20. doi: 10.1128/JVI.00994-20. Print 2020 Jul 30. J Virol. 2020. PMID: 32493817 Free PMC article.
-
Epidemic Zika virus strains from the Asian lineage induce an attenuated fetal brain pathogenicity.Nat Commun. 2024 Dec 30;15(1):10870. doi: 10.1038/s41467-024-55155-4. Nat Commun. 2024. PMID: 39738084 Free PMC article.
-
Congenital Zika Virus Infection Impairs Corpus Callosum Development.Viruses. 2023 Nov 28;15(12):2336. doi: 10.3390/v15122336. Viruses. 2023. PMID: 38140578 Free PMC article.
-
Distinct Replication Kinetics, Cytopathogenicity, and Immune Gene Regulation in Human Microglia Cells Infected with Asian and African Lineages of Zika Virus.Microorganisms. 2024 Sep 5;12(9):1840. doi: 10.3390/microorganisms12091840. Microorganisms. 2024. PMID: 39338514 Free PMC article.
References
-
- Petersen E., Wilson M.E., Touch S., McCloskey B., Mwaba P., Bates M., Dar O., Mattes F., Kidd M., Ippolito G., et al. Rapid Spread of Zika Virus in The Americas--Implications for Public Health Preparedness for Mass Gatherings at the 2016 Brazil Olympic Games. Int J. Infect. Dis. 2016;44:11–15. doi: 10.1016/j.ijid.2016.02.001. - DOI - PubMed
-
- Driggers R.W., Ho C.Y., Korhonen E.M., Kuivanen S., Jaaskelainen A.J., Smura T., Rosenberg A., Hill D.A., DeBiasi R.L., Vezina G., et al. Zika Virus Infection with Prolonged Maternal Viremia and Fetal Brain Abnormalities. N. Engl. J. Med. 2016;374:2142–2151. doi: 10.1056/NEJMoa1601824. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

