Attempt to Untangle the Prion-Like Misfolding Mechanism for Neurodegenerative Diseases
- PMID: 30304819
- PMCID: PMC6213118
- DOI: 10.3390/ijms19103081
Attempt to Untangle the Prion-Like Misfolding Mechanism for Neurodegenerative Diseases
Abstract
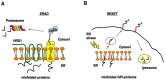
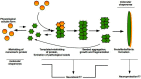
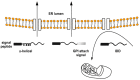
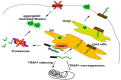
The misfolding and aggregation of proteins is the neuropathological hallmark for numerous diseases including Alzheimer's disease, Parkinson's disease, and prion diseases. It is believed that misfolded and abnormal β-sheets forms of wild-type proteins are the vectors of these diseases by acting as seeds for the aggregation of endogenous proteins. Cellular prion protein (PrPC) is a glycosyl-phosphatidyl-inositol (GPI) anchored glycoprotein that is able to misfold to a pathogenic isoform PrPSc, the causative agent of prion diseases which present as sporadic, dominantly inherited and transmissible infectious disorders. Increasing evidence highlights the importance of prion-like seeding as a mechanism for pathological spread in Alzheimer's disease and Tauopathy, as well as other neurodegenerative disorders. Here, we report the latest findings on the mechanisms controlling protein folding, focusing on the ER (Endoplasmic Reticulum) quality control of GPI-anchored proteins and describe the "prion-like" properties of amyloid-β and tau assemblies. Furthermore, we highlight the importance of pathogenic assemblies interaction with protein and lipid membrane components and their implications in both prion and Alzheimer's diseases.
Keywords: APP; Aβ; PrP; aggregation; amyloid; misfolding; prion protein; prion-like; seeds; tau.
Conflict of interest statement
The author declares no conflict of interest.
Figures
Similar articles
-
Pathogenic mechanisms of prion protein, amyloid-β and α-synuclein misfolding: the prion concept and neurotoxicity of protein oligomers.J Neurochem. 2016 Oct;139(2):162-180. doi: 10.1111/jnc.13772. Epub 2016 Sep 15. J Neurochem. 2016. PMID: 27529376 Review.
-
Amyloidogenesis of Tau protein.Protein Sci. 2017 Nov;26(11):2126-2150. doi: 10.1002/pro.3275. Epub 2017 Sep 13. Protein Sci. 2017. PMID: 28833749 Free PMC article. Review.
-
How an Infection of Sheep Revealed Prion Mechanisms in Alzheimer's Disease and Other Neurodegenerative Disorders.Int J Mol Sci. 2021 May 4;22(9):4861. doi: 10.3390/ijms22094861. Int J Mol Sci. 2021. PMID: 34064393 Free PMC article. Review.
-
Mechanisms and Functions of Spatial Protein Quality Control.Annu Rev Biochem. 2017 Jun 20;86:97-122. doi: 10.1146/annurev-biochem-060815-014616. Epub 2017 May 10. Annu Rev Biochem. 2017. PMID: 28489421 Review.
-
From Seeds to Fibrils and Back: Fragmentation as an Overlooked Step in the Propagation of Prions and Prion-Like Proteins.Biomolecules. 2020 Sep 10;10(9):1305. doi: 10.3390/biom10091305. Biomolecules. 2020. PMID: 32927676 Free PMC article. Review.
Cited by
-
Emerging roles of the cellular prion protein (PrPC) and 37/67 kDa laminin receptor (RPSA) interaction in cancer biology.Cell Mol Life Sci. 2023 Jul 15;80(8):207. doi: 10.1007/s00018-023-04844-2. Cell Mol Life Sci. 2023. PMID: 37452879 Free PMC article. Review.
-
Testing Amyloid Cross-Toxicity in the Vertebrate Brain.ACS Omega. 2020 Jun 15;5(25):15586-15591. doi: 10.1021/acsomega.0c01819. eCollection 2020 Jun 30. ACS Omega. 2020. PMID: 32637834 Free PMC article.
-
Causative Links between Protein Aggregation and Oxidative Stress: A Review.Int J Mol Sci. 2019 Aug 9;20(16):3896. doi: 10.3390/ijms20163896. Int J Mol Sci. 2019. PMID: 31405050 Free PMC article. Review.
-
Endosomal trafficking and related genetic underpinnings as a hub in Alzheimer's disease.J Cell Physiol. 2022 Oct;237(10):3803-3815. doi: 10.1002/jcp.30864. Epub 2022 Aug 22. J Cell Physiol. 2022. PMID: 35994714 Free PMC article. Review.
-
Extracellular vesicles: A new paradigm in understanding, diagnosing and treating neurodegenerative disease.Front Aging Neurosci. 2022 Nov 3;14:967231. doi: 10.3389/fnagi.2022.967231. eCollection 2022. Front Aging Neurosci. 2022. PMID: 36408114 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

