Protective Immune Responses Generated in a Murine Model Following Immunization with Recombinant Schistosoma japonicum Insulin Receptor
- PMID: 30304851
- PMCID: PMC6213549
- DOI: 10.3390/ijms19103088
Protective Immune Responses Generated in a Murine Model Following Immunization with Recombinant Schistosoma japonicum Insulin Receptor
Abstract
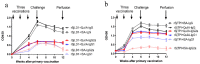
There is a pressing need to develop vaccines for schistosomiasis given the current heavy dependency on praziquantel as the only available drug for treatment. We previously showed the ligand domain of the Schistosoma japonicum insulin receptor 1 and 2 (rSjLD1 and 2) fusion proteins conferred solid protection in mice against challenge infection with S. japonicum. To improve vaccine efficacy, we compared the immunogenicity and protective efficacy of rSjLD1 on its own and in combination with S. japonicum triose-phosphate isomerase (SjTPI), formulated with either of two adjuvants (QuilA and montanide ISA 720VG) in murine vaccine trials against S. japonicum challenge. The level of protection was higher in mice vaccinated only with rSjLD1 formulated with either adjuvant; rSjTPI or the rSjTPI-rSjLD1 combination resulted in a lower level of protection. Mirroring our previous results, there were significant reductions in the number of female worms (30⁻44%), faecal eggs (61⁻68%), liver eggs (44⁻56%), intestinal eggs (46⁻48%) and mature intestinal eggs (58⁻63%) in the rSjLD1-vaccinated mice compared with the adjuvant only groups. At 6-weeks post-cercarial challenge, a significantly increased production of interferon gamma (IFNγ) in rSjLD1-stimulated splenic CD4⁺ T cells was observed in the rSjLD1-vaccinated mice suggesting a Th1-type response is associated with the generated level of protective efficacy.
Keywords: Schistosoma japonicum; insulin receptor; murine model; triose-phosphate isomerase; vaccine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Enhancement of protective efficacy through adenoviral vectored vaccine priming and protein boosting strategy encoding triosephosphate isomerase (SjTPI) against Schistosoma japonicum in mice.PLoS One. 2015 Mar 20;10(3):e0120792. doi: 10.1371/journal.pone.0120792. eCollection 2015. PLoS One. 2015. PMID: 25793406 Free PMC article.
-
Evaluation of protective efficacy induced by different heterologous prime-boost strategies encoding triosephosphate isomerase against Schistosoma japonicum in mice.Parasit Vectors. 2017 Feb 28;10(1):111. doi: 10.1186/s13071-017-2036-5. Parasit Vectors. 2017. PMID: 28241779 Free PMC article.
-
SjHSP70, a recombinant Schistosoma japonicum heat shock protein 70, is immunostimulatory and induces protective immunity against cercarial challenge in mice.Parasitol Res. 2015 Sep;114(9):3415-29. doi: 10.1007/s00436-015-4567-z. Epub 2015 Jun 21. Parasitol Res. 2015. PMID: 26091761
-
A vaccine against the Asian schistosome, Schistosoma japonicum: an update on paramyosin as a target of protective immunity.Int J Parasitol. 1997 Oct;27(10):1213-9. doi: 10.1016/s0020-7519(97)00119-7. Int J Parasitol. 1997. PMID: 9394192 Review.
-
Infection characteristics of Schistosoma japonicum in mice and relevance to the assessment of schistosome vaccines.Adv Parasitol. 1991;30:167-200. doi: 10.1016/s0065-308x(08)60308-5. Adv Parasitol. 1991. PMID: 1906234 Review. No abstract available.
Cited by
-
Protein Kinases: Potential Drug Targets Against Schistosoma japonicum.Front Cell Infect Microbiol. 2021 Jul 1;11:691757. doi: 10.3389/fcimb.2021.691757. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 34277472 Free PMC article. Review.
-
Transmission-Blocking Vaccines against Schistosomiasis Japonica.Int J Mol Sci. 2024 Jan 30;25(3):1707. doi: 10.3390/ijms25031707. Int J Mol Sci. 2024. PMID: 38338980 Free PMC article. Review.
-
Duplex real-time PCR for sexing Schistosoma japonicum cercariae based on W chromosome-specific genes and its applications.PLoS Negl Trop Dis. 2020 Aug 21;14(8):e0008609. doi: 10.1371/journal.pntd.0008609. eCollection 2020 Aug. PLoS Negl Trop Dis. 2020. PMID: 32822351 Free PMC article.
-
Vaccines for Human Schistosomiasis: Recent Progress, New Developments and Future Prospects.Int J Mol Sci. 2022 Feb 18;23(4):2255. doi: 10.3390/ijms23042255. Int J Mol Sci. 2022. PMID: 35216369 Free PMC article. Review.
-
The Search for a Schistosomiasis Vaccine: Australia's Contribution.Vaccines (Basel). 2021 Aug 6;9(8):872. doi: 10.3390/vaccines9080872. Vaccines (Basel). 2021. PMID: 34451997 Free PMC article. Review.
References
-
- Gordon C.A., Acosta L.P., Gray D.J., Olveda R.M., Jarilla B., Gobert G.N., Ross A.G., McManus D.P. High prevalence of Schistosoma japonicum infection in Carabao from Samar Province, the Philippines: Implications for transmission and control. PLoS Negl. Trop. Dis. 2012;6:e1778. doi: 10.1371/journal.pntd.0001778. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

