The Reelin Receptors Apolipoprotein E receptor 2 (ApoER2) and VLDL Receptor
- PMID: 30304853
- PMCID: PMC6213145
- DOI: 10.3390/ijms19103090
The Reelin Receptors Apolipoprotein E receptor 2 (ApoER2) and VLDL Receptor
Abstract
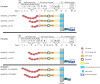
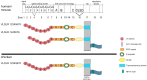
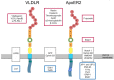
Apolipoprotein E receptor 2 (ApoER2) and VLDL receptor belong to the low density lipoprotein receptor family and bind apolipoprotein E. These receptors interact with the clathrin machinery to mediate endocytosis of macromolecules but also interact with other adapter proteins to perform as signal transduction receptors. The best characterized signaling pathway in which ApoER2 and VLDL receptor (VLDLR) are involved is the Reelin pathway. This pathway plays a pivotal role in the development of laminated structures of the brain and in synaptic plasticity of the adult brain. Since Reelin and apolipoprotein E, are ligands of ApoER2 and VLDLR, these receptors are of interest with respect to Alzheimer's disease. We will focus this review on the complex structure of ApoER2 and VLDLR and a recently characterized ligand, namely clusterin.
Keywords: Alzheimer’s disease; VLDL receptor; apolipoprotein E receptor 2; clusterin; reelin.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Trommsdorff M., Gotthardt M., Hiesberger T., Shelton J., Stockinger W., Nimpf J., Hammer R.E., Richardson J.A., Herz J. Reeler/disabled-like disruption of neuronal migration in knockout mice lacking the VLDL receptor and ApoE receptor 2. Cell. 1999;97:689–701. doi: 10.1016/S0092-8674(00)80782-5. - DOI - PubMed
-
- Kim D.-H., Iijima H., Goto K., Sakai J., Ishii H., Kim H.-J., Suzuki H., Kondo H., Saeki S., Yamamoto T. Human apolipoprotein E receptor 2. A novel lipoprotein receptor of the low density lipoprotein receptor family predominantly expressed in brain. J. Biol. Chem. 1996;271:8373–8380. doi: 10.1074/jbc.271.14.8373. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

