Antizyme Inhibitors in Polyamine Metabolism and Beyond: Physiopathological Implications
- PMID: 30304856
- PMCID: PMC6313458
- DOI: 10.3390/medsci6040089
Antizyme Inhibitors in Polyamine Metabolism and Beyond: Physiopathological Implications
Abstract
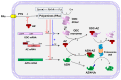
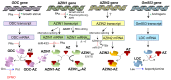
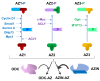
The intracellular levels of polyamines, cationic molecules involved in a myriad of cellular functions ranging from cellular growth, differentiation and apoptosis, is precisely regulated by antizymes and antizyme inhibitors via the modulation of the polyamine biosynthetic and transport systems. Antizymes, which are mainly activated upon high polyamine levels, inhibit ornithine decarboxylase (ODC), the key enzyme of the polyamine biosynthetic route, and exert a negative control of polyamine intake. Antizyme inhibitors (AZINs), which are proteins highly homologous to ODC, selectively interact with antizymes, preventing their action on ODC and the polyamine transport system. In this review, we will update the recent advances on the structural, cellular and physiological functions of AZINs, with particular emphasis on the action of these proteins in the regulation of polyamine metabolism. In addition, we will describe emerging evidence that suggests that AZINs may also have polyamine-independent effects on cells. Finally, we will discuss how the dysregulation of AZIN activity has been implicated in certain human pathologies such as cancer, fibrosis or neurodegenerative diseases.
Keywords: antizyme; antizyme inhibitors; ornithine decarboxylase; polyamine metabolism; polyamines.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
References
-
- Pegg A.E. Polyamine metabolism and its importance in neoplastic growth and a target for chemotherapy. Cancer Res. 1988;48:759–774. - PubMed
-
- Cason A.L., Ikeguchi Y., Skinner C., Wood T.C., Holden K.R., Lubs H.A., Martinez F., Simensen R.J., Stevenson R.E., Pegg A.E., et al. X-linked spermine synthase gene (SMS) defect: The first polyamine deficiency syndrome. Eur. J. Hum. Genet. 2003;11:937–944. doi: 10.1038/sj.ejhg.5201072. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

