Targeted Theranostic Nanoparticles for Brain Tumor Treatment
- PMID: 30304861
- PMCID: PMC6321593
- DOI: 10.3390/pharmaceutics10040181
Targeted Theranostic Nanoparticles for Brain Tumor Treatment
Abstract
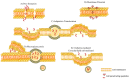
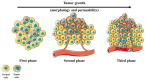
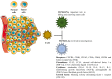
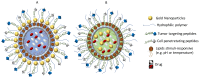
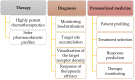
The poor prognosis and rapid recurrence of glioblastoma (GB) are associated to its fast-growing process and invasive nature, which make difficult the complete removal of the cancer infiltrated tissues. Additionally, GB heterogeneity within and between patients demands a patient-focused method of treatment. Thus, the implementation of nanotechnology is an attractive approach considering all anatomic issues of GB, since it will potentially improve brain drug distribution, due to the interaction between the blood⁻brain barrier and nanoparticles (NPs). In recent years, theranostic techniques have also been proposed and regarded as promising. NPs are advantageous for this application, due to their respective size, easy surface modification and versatility to integrate multiple functional components in one system. The design of nanoparticles focused on therapeutic and diagnostic applications has increased exponentially for the treatment of cancer. This dual approach helps to understand the location of the tumor tissue, the biodistribution of nanoparticles, the progress and efficacy of the treatment, and is highly useful for personalized medicine-based therapeutic interventions. To improve theranostic approaches, different active strategies can be used to modulate the surface of the nanotheranostic particle, including surface markers, proteins, drugs or genes, and take advantage of the characteristics of the microenvironment using stimuli responsive triggers. This review focuses on the different strategies to improve the GB treatment, describing some cell surface markers and their ligands, and reports some strategies, and their efficacy, used in the current research.
Keywords: active targeting; glioblastoma; gold nanoparticles; lipid nanoparticles; nanotechnology; theranostics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Louis D.N., Perry A., Reifenberger G., Von Deimling A., Figarella-Branger D., Cavenee W.K., Ohgaki H., Wiestler O.D., Kleihues P., Ellison D.W. The 2016 World Health Organization classification of tumors of the central nervous system: A summary. Acta Neuropathol. 2016;131:803–820. doi: 10.1007/s00401-016-1545-1. - DOI - PubMed
-
- Chandana S.R., Movva S., Arora M., Singh T. Primary brain tumors in adults. Am. Fam. Physician. 2008;77:1423–1430. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

