Feline low-grade alimentary lymphoma: an emerging entity and a potential animal model for human disease
- PMID: 30305106
- PMCID: PMC6180644
- DOI: 10.1186/s12917-018-1635-5
Feline low-grade alimentary lymphoma: an emerging entity and a potential animal model for human disease
Abstract
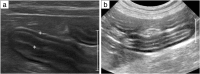
Background: Low-grade alimentary lymphoma (LGAL) is characterised by the infiltration of neoplastic T-lymphocytes, typically in the small intestine. The incidence of LGAL has increased over the last ten years and it is now the most frequent digestive neoplasia in cats and comprises 60 to 75% of gastrointestinal lymphoma cases. Given that LGAL shares common clinical, paraclinical and ultrasonographic features with inflammatory bowel diseases, establishing a diagnosis is challenging. A review was designed to summarise current knowledge of the pathogenesis, diagnosis, prognosis and treatment of feline LGAL. Electronic searches of PubMed and Science Direct were carried out without date or language restrictions.
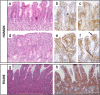
Results: A total of 176 peer-reviewed documents were identified and most of which were published in the last twenty years. 130 studies were found from the veterinary literature and 46 from the human medicine literature. Heterogeneity of study designs and outcome measures made meta-analysis inappropriate. The pathophysiology of feline LGAL still needs to be elucidated, not least the putative roles of infectious agents, environmental factors as well as genetic events. The most common therapeutic strategy is combination treatment with prednisolone and chlorambucil, and prolonged remission can often be achieved. Developments in immunohistochemical analysis and clonality testing have improved the confidence of clinicians in obtaining a correct diagnosis between LGAL and IBD. The condition shares similarities with some diseases in humans, especially human indolent T-cell lymphoproliferative disorder of the gastrointestinal tract.
Conclusions: The pathophysiology of feline LGAL still needs to be elucidated and prospective studies as well as standardisation of therapeutic strategies are needed. A combination of conventional histopathology and immunohistochemistry remains the current gold-standard test, but clinicians should be cautious about reclassifying cats previously diagnosed with IBD to lymphoma on the basis of clonality testing. Importantly, feline LGAL could be considered to be a potential animal model for indolent digestive T-cell lymphoproliferative disorder, a rare condition in human medicine.
Keywords: Cat; Comparative oncology; Human indolent digestive T-cell lymphoproliferative disorder; Inflammatory bowel disease.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
AJG’s academic post at the University of Liverpool is financially supported by Royal Canin. Otherwise, the authors declare that they had no conflicts of interests with respect to their authorship or the publication of this article.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures



References
-
- Twomey LN, Compendium AA. Cytodiagnosis of feline lymphoma. Compendium. 2005;27:1.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

