Association between fluid management and dilutional coagulopathy in severe postpartum haemorrhage: a nationwide retrospective cohort study
- PMID: 30305108
- PMCID: PMC6180574
- DOI: 10.1186/s12884-018-2021-9
Association between fluid management and dilutional coagulopathy in severe postpartum haemorrhage: a nationwide retrospective cohort study
Abstract
Background: The view that 2 l of crystalloid and 1.5 l of colloid can be infused while awaiting compatible blood for patients with major postpartum haemorrhage is based on expert opinion documents. We describe real-world changes in levels of coagulation parameters after the administration of different volumes of clear fluids to women suffering from major postpartum haemorrhage.
Methods: We performed a nationwide retrospective cohort study in the Netherlands among 1038 women experiencing severe postpartum haemorrhage who had received at least four units of red cells or fresh frozen plasma or platelets in addition to red cells. The volume of clear fluids administered before the time of blood sampling was classified into three fluid administration strategies, based on the RCOG guideline: < 2 L, 2-3.5 L and > 3.5 L. Outcomes included haemoglobin, haematocrit, platelet count, fibrinogen, aPTT and PT levels.
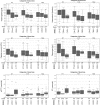
Results: Haemoglobin, haematocrit, platelet count, fibrinogen and aPTT were associated with volumes of clear fluids, which was most pronounced early during the course of postpartum haemorrhage. During the earliest phases of postpartum haemorrhage median haemoglobin level was 10.1 g/dl (IQR 8.5-11.6) among the women who received < 2 L clear fluids and 8.1 g/dl (IQR 7.1-8.4) among women who received > 3.5 L of clear fluids; similarly median platelet counts were 181 × 109/litre (IQR 131-239) and 89 × 109/litre (IQR 84-135), aPTT 29 s (IQR 27-33) and 38 s (IQR 35-55) and fibrinogen 3.9 g/L (IQR 2.5-5.2) and 1.6 g/L (IQR 1.3-2.1).
Conclusions: In this large cohort of women with severe postpartum haemorrhage, administration of larger volumes of clear fluids was associated with more severe deterioration of coagulation parameters corresponding to dilution. Our findings provide thus far the best available evidence to support expert opinion-based guidelines recommending restrictive fluid resuscitation in women experiencing postpartum haemorrhage.
Trial registration: Netherlands Trial Register ( NTR4079 ), registration date July 17, 2013.
Keywords: Coagulation parameters; Dilutional coagulopathy; Fluid management; Postpartum haemorrhage.
Conflict of interest statement
Ethics approval and consent to participate
The Ethical Committee of Leiden University Medical Centre (P12.273) and the institutional review boards of all participating hospitals approved of the study. The study was registered in the Netherlands Trial Register (NTR4079). The need to obtain informed consent was waived by the ethics committee because of the retrospective design.
Consent for publication
Not applicable.
Competing interests
One of the co-authors of this manuscript, Jos J. M. van Roosmalen is a member of the editorial board (Section Editor) of BMC pregnancy and childbirth.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

