The LEAD trial - the effectiveness of a decision aid on decision making among citizens with lower educational attainment who have not participated in FIT-based colorectal cancer screening in Denmark: study protocol for a randomized controlled trial
- PMID: 30305114
- PMCID: PMC6180588
- DOI: 10.1186/s13063-018-2921-z
The LEAD trial - the effectiveness of a decision aid on decision making among citizens with lower educational attainment who have not participated in FIT-based colorectal cancer screening in Denmark: study protocol for a randomized controlled trial
Abstract
Background: Colorectal cancer screening participation is a preference-sensitive choice, in which trade-offs between benefits and harms must be made by individual citizens. Often the decision is made without any contact with healthcare professionals. Citizens with lower educational attainment tend to participate less in colorectal cancer screening than citizens with average educational attainment. Further, they tend to have lower levels of knowledge about colorectal cancer screening. Providing lower educational attainment citizens with a targeted decision aid embracing their diverse information needs might increase these citizens' ability to make informed decisions. The aim of this trial is to test the effectiveness of such a newly developed self-administered decision aid.
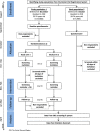
Methods: The LEAD (Lower Educational Attainment Decision aid) trial will be conducted as a two-arm randomized controlled trial among 10,000 50-74-year-old citizens, resident in the Central Denmark Region not yet invited to take up colorectal cancer screening. Citizens will receive a baseline questionnaire. Respondents will be allocated into the intervention or the control groups. Citizens in the intervention group will receive the decision aid whereas the control group will not. Those who return a stool sample within 45 days after receiving the screening invitation and those with medium or higher educational attainment are excluded. Both groups will receive a follow-up questionnaire 90 days after being invited to colorectal cancer screening. A historic cohort consisting of 5000 50-74-year-old citizens resident in the Central Denmark Region, having received their screening invitation in the beginning of 2017 will be included. This cohort will receive a follow-up questionnaire 6-9 months after they received the screening invitation. Informed choice will be evaluated by assessing levels of knowledge, attitudes, and screening uptake. Analyses will be conducted as intention-to-treat analyses. Additionally, differences between levels of worry and decisional conflict between groups will be assessed as secondary outcomes.
Discussion: This trial will evaluate whether a targeted decision aid is a feasible way of enhancing informed choice among lower educational attainment citizens in colorectal cancer screening. Further, it may guide decisions about providing information material in cancer screening in general.
Trial registration: ClinicalTrials.gov, NCT03253888 . Registered on 17 August 2017.
Keywords: Cancer screening; Colorectal cancer; Decision aid; Lower educational attainment; Randomized controlled trial.
Conflict of interest statement
Ethics approval and consent to participate
The Danish Data Protection Agency gave permission to collect and hold survey data and obtain registry data (J.no.: 2012-58-006 / Case no.: 1-16-02-94-16). Further the study achieved clearance at The Danish Patient Safety Authorities (J.no.: 3-313-1729-1) and the Central Denmark Region Committees on Health Research Ethics (143/2016). The trial is registered in ClinicalTrials.gov (NCT03253888) (see Additional file 4).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
Effectiveness of a decision aid for colorectal cancer screening on components of informed choice according to educational attainment: A randomised controlled trial.PLoS One. 2020 Nov 10;15(11):e0241703. doi: 10.1371/journal.pone.0241703. eCollection 2020. PLoS One. 2020. PMID: 33170877 Free PMC article. Clinical Trial.
-
The LEAD trial-The effectiveness of a decision aid on decision making among citizens with lower educational attainment who have not participated in FIT-based colorectal cancer screening in Denmark: A randomised controlled trial.Patient Educ Couns. 2020 Feb;103(2):359-368. doi: 10.1016/j.pec.2019.08.029. Epub 2019 Aug 19. Patient Educ Couns. 2020. PMID: 31451360 Clinical Trial.
-
Making decisions about colorectal cancer screening. A qualitative study among citizens with lower educational attainment.Eur J Public Health. 2016 Feb;26(1):176-81. doi: 10.1093/eurpub/ckv207. Epub 2015 Nov 4. Eur J Public Health. 2016. PMID: 26541860
-
A Personalized Decision Aid to Help Women with Lupus Nephritis from Racially and Ethnically Diverse Backgrounds Make Decisions about Taking Immune-Blocking Medicines [Internet].Washington (DC): Patient-Centered Outcomes Research Institute (PCORI); 2019 Oct. Washington (DC): Patient-Centered Outcomes Research Institute (PCORI); 2019 Oct. PMID: 37262201 Free Books & Documents. Review.
-
A Patient Decision Aid to Help Heavy Smokers Make Decisions about Lung Cancer Screening [Internet].Washington (DC): Patient-Centered Outcomes Research Institute (PCORI); 2019 Jun. Washington (DC): Patient-Centered Outcomes Research Institute (PCORI); 2019 Jun. PMID: 38620344 Free Books & Documents. Review.
Cited by
-
Effectiveness of a decision aid for colorectal cancer screening on components of informed choice according to educational attainment: A randomised controlled trial.PLoS One. 2020 Nov 10;15(11):e0241703. doi: 10.1371/journal.pone.0241703. eCollection 2020. PLoS One. 2020. PMID: 33170877 Free PMC article. Clinical Trial.
-
Systematic Development of Patient Decision Aids: An Update from the IPDAS Collaboration.Med Decis Making. 2021 Oct;41(7):736-754. doi: 10.1177/0272989X211014163. Epub 2021 Jun 19. Med Decis Making. 2021. PMID: 34148384 Free PMC article.
References
-
- Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet] Lyon: International Agency for Research on Cancer; 2013.
-
- Fransen MP, Dekker E, Timmermans DR, Uiters E, Essink-Bot ML. Accessibility of standardized information of a national colorectal cancer screening program for low health literate screening invitees: A mixed method study. Patient Educ Couns. 2017;100:327–336. doi: 10.1016/j.pec.2016.09.004. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

