Short-term physiological hypoxia potentiates the therapeutic function of mesenchymal stem cells
- PMID: 30305185
- PMCID: PMC6180371
- DOI: 10.1186/s13287-018-1007-x
Short-term physiological hypoxia potentiates the therapeutic function of mesenchymal stem cells
Abstract
Background: In the bone marrow, MSCs reside in a hypoxic milieu (1-5% O2) that is thought to preserve their multipotent state. Typically, in vitro expansion of MSCs is performed under normoxia (~ 21% O2), a process that has been shown to impair their function. Here, we evaluated the characteristics and function of MSCs cultured under hypoxia and hypothesized that, when compared to normoxia, dedicated hypoxia will augment the functional characteristics of MSCs.
Methods: Human and porcine bone marrow MSCs were obtained from fresh mononuclear cells. The first study evaluated MSC function following both long-term (10 days) and short-term (48 h) hypoxia (1% O2) culture. In our second study, we evaluated the functional characteristics of MSC cultured under short-term 2% and 5% hypoxia. MSCs were evaluated for their metabolic activity, proliferation, viability, clonogenicity, gene expression, and secretory capacity.
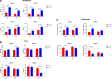
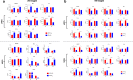
Results: In long-term culture, common MSC surface marker expression (CD44 and CD105) dropped under hypoxia. Additionally, in long-term culture, MSCs proliferated significantly slower and provided lower yields under hypoxia. Conversely, in short-term culture, MSCs proliferated significantly faster under hypoxia. In both long-term and short-term cultures, MSC metabolic activity was significantly higher under hypoxia. Furthermore, MSCs cultured under hypoxia had upregulated expression of VEGF with concomitant downregulation of HMGB1 and the apoptotic genes BCL-2 and CASP3. Finally, in both hypoxia cultures, the pro-inflammatory cytokine, IL-8, was suppressed, while levels of the anti-inflammatories, IL-1ra and GM-CSF, were elevated in short-term hypoxia only.
Conclusions: In this study, we demonstrate that hypoxia augments the therapeutic characteristics of both porcine and human MSCs. Yet, short-term 2% hypoxia offers the greatest benefit overall, exemplified by the increase in proliferation, self-renewing capacity, and modulation of key genes and the inflammatory milieu as compared to normoxia. These data are important for generating robust MSCs with augmented function for clinical applications.
Keywords: Bone marrow; Hypoxia; Immunomodulation; Mesenchymal stem cells (MSCs); Normoxia.
Conflict of interest statement
Ethics approval and consent to participate
N/A
Consent for publication
N/A
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

